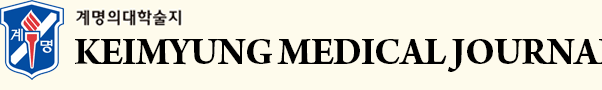|
 |
- Search
| Keimyung Med J > Volume 42(2); 2023 > Article |
|
Abstract
Solitary fibrous tumor (SFT) is an uncommon tumor that typically originates in the pleural cavity but may also be found in extra-pleura sites like the head and neck, spine, lungs, mediastinum, peritoneum, and pelvis. There are few reports of SFT of the scalp, and malignant SFT of the scalp appears to be so rare that only two cases of it were reported previously. A 47-year-old woman was admitted to our institution with a large, rapidly growing mass on the left parietal scalp. An analysis of her scalp condition and imaging findings of the mass revealed two different appearances between the superior and inferior halves; the scalp of the inferior half looked normal, but that of the superior half did not. Also, computed tomography findings of the mass confirmed different patterns between the superior and inferior halves. The mass with the abnormal superior half scalp was removed totally. The pathological diagnosis was malignant SFT, but the inferior mass was revealed to have a benign nature. The patient underwent prophylactic radiotherapy and experienced no local recurrence or distant metastasis at the final follow-up at 12 months. We present a third case of malignant SFT originating on the scalp and describe our clinical and surgical experience managing malignant SFT of the scalp.
Solitary fibrous tumor (SFT) is an uncommon mesenchymal cell tumor that usually originates in the pleural cavity. However, extra-pleura sites of tumor origin include the head and neck, spine, lungs, mediastinum, peritoneum, and pelvis [1-5]. Although SFT has a generally benign nature, its variable clinical course encompasses local invasion and multiple metastases. The pathological characteristics of SFT include nodular spindle-cell proliferation, and specific immunoreactive stains are helpful for diagnosis. Among the various sites of origin, SFT originating from the scalp has been reported in 16 cases [6-9]. In contrast, malignant SFTs with a scalp origin are extremely rare, with only two cases reported in the literature [10,11].
We present a rare case of malignant SFT originating from the scalp, which appeared to be rapidly growing and huge. In this report, we describe our experience with the clinical presentation, imaging findings, pathological characteristics, and surgical outcome of malignant SFT of the scalp.
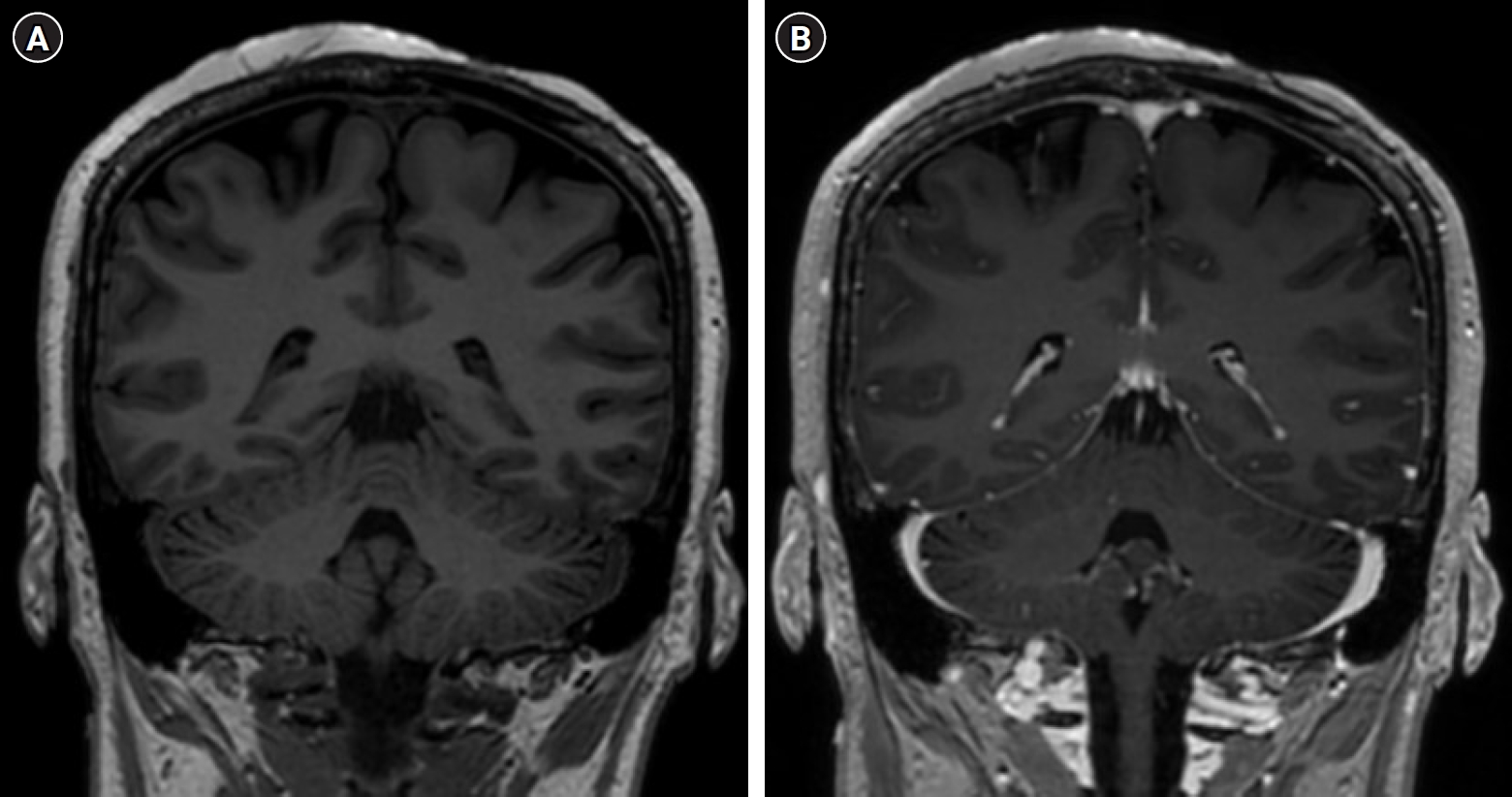
A 47-year-old woman was admitted to our institution with a huge mass on the left parietal scalp. The patient had experienced a minor head trauma (collision with the edge of a table) five years prior, and her scalp had swelled at the time of injury. Over time, the swelling of the scalp decreased slightly, but did not resolve completely, and a scalp mass persisted for a few months. She reported that the scalp mass had been growing very slowly until recently. The patient stated that the scalp mass was initially a dome-shaped mass measuring about 5 cm in size. Four months prior to the current presentation, however, the lesion had started to rapidly increase in size. Upon examination, the mass appeared to have a cylindrical shape in the vertical direction. The maximum size of the mass was about 8 × 7 × 11 cm grossly, and it was hard, smooth, painless, and not tender on palpation. There was no abnormal pulsation on the scalp over the mass. The surface of the scalp was divided into superior and inferior halves according to differences in appearance (Fig. 1). The superior half of the scalp appeared to be very thin and pale, with no hair. There were many dilated vessels and disruptions of the normal epidermal layer and ulceration. Conversely, the inferior half of the scalp had a normal appearance with hair, hair follicles, and normal coloring. The patient did not have a previous history of infection. No focal neurologic deficit was found. The laboratory values of the patient were within normal ranges.
Computed tomography (CT) imaging demonstrated that the mass was well circumscribed with heterogeneous density on non-enhanced images, and the size of the mass was about 10.6 cm in height and 7.4 × 6.5 cm in diameter. The enhanced pattern of the mass was different between the superior and inferior halves of the scalp (Fig. 2). The inferior portion of the mass included a heterogeneous but well-enhanced lesion on the peripheral portion with a less-enhanced central portion. The superior half of the mass was less enhanced compared to the inferior half and had a low density of the central portion. The left parietal bone attached under the mass showed wide bony erosion but no bone invasion. The thickness of the inferior half of the scalp was the same as that of the normal skull. However, the superior half of the scalp was very thin due to compression by a huge mass. There was no intracranial lesion, such as local invasion or a metastatic mass. To assess the patient for systemic disease, positron emission tomography was performed and demonstrated no other uptake lesions throughout the body.
Surgery was performed under general anesthesia with the patient in the supine position with neck flexion. An incision was created between the abnormal superior and normal inferior scalp margins to remove the abnormal scalp with the superior half of the mass. After a circumferential incision of the scalp was made, the superior portion of the mass was easily detached from the inferior mass and removed together with attached abnormal scalp skin in a single piece (Fig. 3A). The remaining inferior portion of the mass was adhered to the scalp by a fibrotic tissue layer but was well-demarcated, and we easily dissected it from the scalp without any evidence of invasion of surrounding tissue (Fig. 3B). The frozen section of a superior portion of the mass revealed a malignant tumor with suspicion of SFT. The left parietal bone was very thin but had clear margins and no invasion. After removal of the entire mass, there was a scalp defect of the same size as the mass. The defect was closed by a plastic surgeon using remnant normal inferior scalp tissue that had been overlying the removed mass.
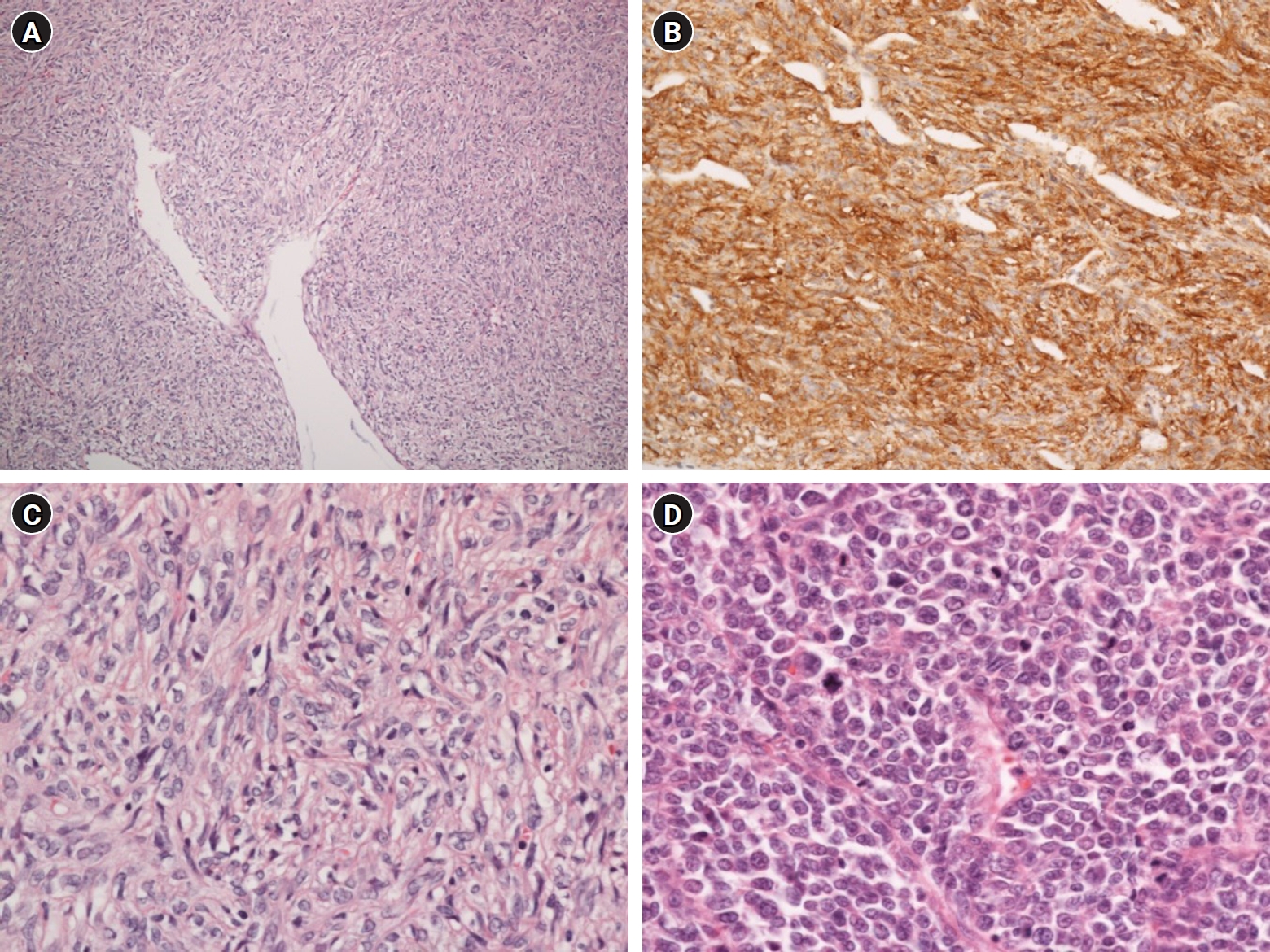
The final pathologic report was malignant SFT. A pleomorphic spindle-cell proliferation with branching and hyalinized “staghorn”-shaped blood vessels in variable collagenous stroma was revealed by microscopic examination (Fig. 4). CD34 and vimentin were positive in immunohistochemical stains. However, desmin, smooth muscle actin, S-100, myogenin, CD99, and myo D1 were negative. The inferior and superior portions of the mass have different features: the well-enhanced inferior mass had a benign nature with a hypocellular appearance and fewer pleomorphic cells, while the less-enhanced and superior mass displayed malignant features with a hypercellular nature, high mitotic figure (> 10 mitoses/10 high-powered fields), and areas of necrosis.
We delivered prophylactic radiotherapy (radiation dose, 60 Gy/30 fx) to the operation field at four weeks after resection. At the final follow-up of 12 months, there was no local recurrence or intracranial metastasis on magnetic resonance imaging (MRI) (Fig. 5).
SFT was first reported as a pleural tumor in 1931 by Klemperer and Rabin [12]. The etiology of SFT is unclear but is thought to originate from mesenchymal cells [13]. Hemangiopericytoma, which is a tumor also with a mesenchymal origin, was considered a distinct neoplasm previously [14]. However, SFT and hemangiopericytoma were combined and reclassified as the same entity (SFT/hemangiopericytoma) in the 2016 World Health Organization classification. Contrary to recognizing that SFT is a pleural neoplasm initially, it can arise at various sites, such as head and neck, spine, lungs, mediastinum, peritoneum, pelvis, genitourinary system, skin, and liver [1-5,15,16]. Almost all SFT cases are benign, but about 6.5% of SFT cases are be malignant [17]. SFT with a scalp origin is very rare, with just 16 cases reported previously [6-9,18-21]. As with SFT at other sites, malignant SFTs of the scalp are rare, with just two cases reported. The first case was reported by Shirley et al. and presented a patient with a right parietal scalp mass that was resected completely and diagnosed as malignant SFT [11]. The second case was reported by Rabie et al. and involved a recurrent malignant SFT on the occipital scalp that was diagnosed as dermatofibrosarcoma after the first operation; however, the recurrent mass was diagnosed as malignant SFT [10]. To the best of our knowledge, our case is the third of malignant SFT of the scalp.
Histological characteristics of SFT are based on a “staghorn” pattern of branching vessels and spindle cell proliferation. Because of the difficult diagnosis of SFT by histologic features due to morphological variation, immunohistochemistry staining is a valuable tool. SFT is stained for CD34, CD99, Bcl-2, and activator of transcription 6 (STAT6), with variable or negative stain for cytokeratins, smooth muscle actin, epithelial membrane antigen, desmin, and S100 [22]. Especially, STAT6 is a highly sensitive and specific marker for SFT diagnosis [23]. Malignant SFT could be distinguished from benign SFT using several factors including mitotic activity (> 4 mitoses/10 high-powered fields [HPF]), mass size > 5 cm, degree of cellularity, presence of immature and pleomorphic tumor cells, and hemorrhage and necrosis [24]. In our case, the pathological features of the inferior mass were low cellularity, few pleomorphic cells, and low mitotic activity. However, the superior mass had different pathologic features, such as high cellularity and > 10 mitoses/10 HPFs. So, both benign and malignant pathologies were observed in this single case. Scalp SFT clinically mimics lipoma and epidermoid cysts. Pathological differential diagnoses for malignant SFT on the scalp include dermatofibrosarcoma protuberans, synovial sarcoma, liposarcoma, and leiomyosarcoma [25,26]. Dermatofibrosarcoma protuberans is a rare soft tissue tumor located commonly on the trunk. The tumor usually involves the dermis and subcutaneous fat. Synovial sarcoma is a malignant tumor involving muscle and ligaments and presents in the arm and leg near the joints. Liposarcoma is a rare tumor originated from fatty tissue, usually in the abdomen or thigh. Leiomyosarcoma is a rare malignant tumor involving smooth muscles. It presents commonly in the intestines, stomach, and blood vessels. The radiologic findings of these malignant soft tissue tumors, which are uncommon on the scalp, are generally irregular with no well-circumscribed margin and frequent local invasion. However, differential diagnosis based on radiological findings was not found in the literature review.
There were no definite radiological findings of SFT on CT or MRI. In general, like other subcutaneous tumors, benign SFT is well-demarcated with a lobulated contour and usually presents with absence of local invasion. However, a malignant SFT has a not well-defined margin and usually demonstrates local invasion. In our case, the peripheral portion of the inferior mass was diagnosed as benign SFT and the superior portion of the mass was diagnosed as malignant SFT pathologically. On CT imaging, the mass appeared entirely well-demarcated, but the peripheral portion of the inferior mass was only well-enhanced, and the central portions of the inferior and superior masses were not enhanced. The non-enhanced portion of the inferior mass had a connection to the superior mass at the upper border of the inferior mass. It may be that the superior mass was arising from the center of the inferior mass and grew upward to form the superior mass. On consideration of the clinical course of mass growth (rapid growth for four months after slow growth) and the difference between the inferior and superior halves of the scalp (the inferior half appeared normal due to slow growth of the mass, but the superior scalp was abnormal due to the fast-growing mass), the belief is that the superior mass was a malignant transformation from a benign inferior mass.
Treatment choices for malignant SFT include complete surgical resection. Meanwhile, adjuvant chemotherapy and radiotherapy of malignant SFT after surgical resection are controversial. Cox et al. suggested that complete resection of head and neck SFT with a clear margin does not require additional treatment and had a good prognosis [17]. Pisters et al. reported the good response to radiotherapy of malignant SFT with > 50 Gy of radiation [27]. There are some reports about the benefits of chemotherapy for malignant SFT [28]. However, because of the lack of cases, the role of adjuvant therapy after surgical resection is not obvious. Yang et al. reported a recurrence rate of 11% and a metastasis rate of 33% [29]. There is also a report that the number of mitoses correlates with prognosis [30]. However, because of the few cases of malignant SFT and the lack of studies on SFT in general, it is difficult to determine the clinical prognosis, and long-term follow-up is necessary. In both previous cases of malignant SFT on the scalp, neither received adjuvant treatment. Although the follow-up durations were 18 and 6 months, respectively, no recurrence or distant metastasis was found. In our case, malignant SFT on the scalp was dissected easily with adjacent tissue to obtain complete resection, and we performed adjuvant radiotherapy. At the final follow-up at 12 months, there was no local recurrence or distant metastasis.
Malignant SFT of the scalp is an extremely rare tumor. To our knowledge, this is the third case of malignant SFT of the scalp. Complete surgical resection is the preferred treatment, while the role of adjuvant treatment is not obvious yet. To date, the number of studies is insufficient, and the follow-up period is typically short. A large number of patients and long-term follow-up are needed to determine the surgical results of malignant SFT of the scalp.
Notes
Fig. 1.
Different appearance of the superior and inferior halve of the scalp. The superior half of the scalp was pale with no hair, many dilated vessels, disruptions of skin and ulceration. The inferior half of the scalp had a normal appearance.
Fig. 2.
Different enhanced pattern of the superior and inferior mass on non-enhanced (A) and enhanced (B) coronal computed tomography image. A heterogeneous but well-enhanced lesion on the peripheral portion with a less-enhanced central portion of the inferior portion of the mass. The superior half of the mass was less enhanced compared to the inferior half.
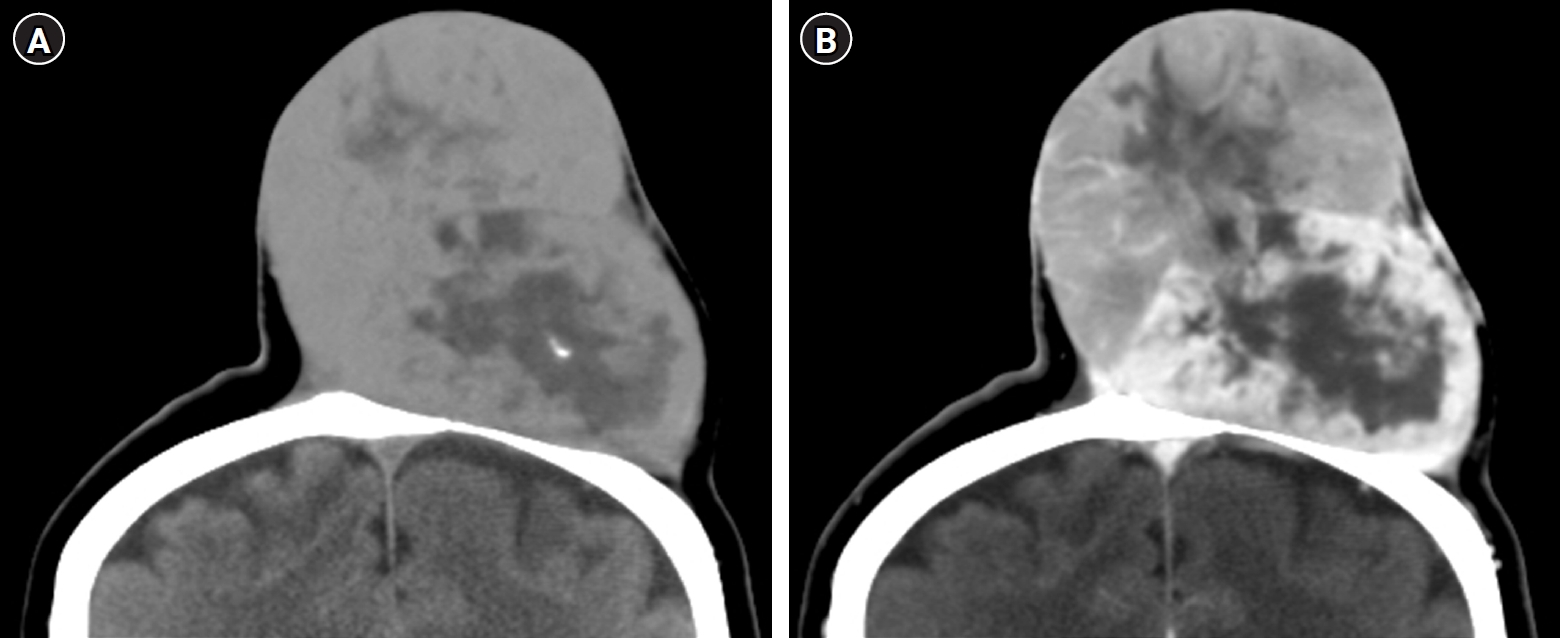
Fig. 3.
The resected superior portion of mass with abnormal scalp (A). Well-demarcated inferior portion of the mass (B).
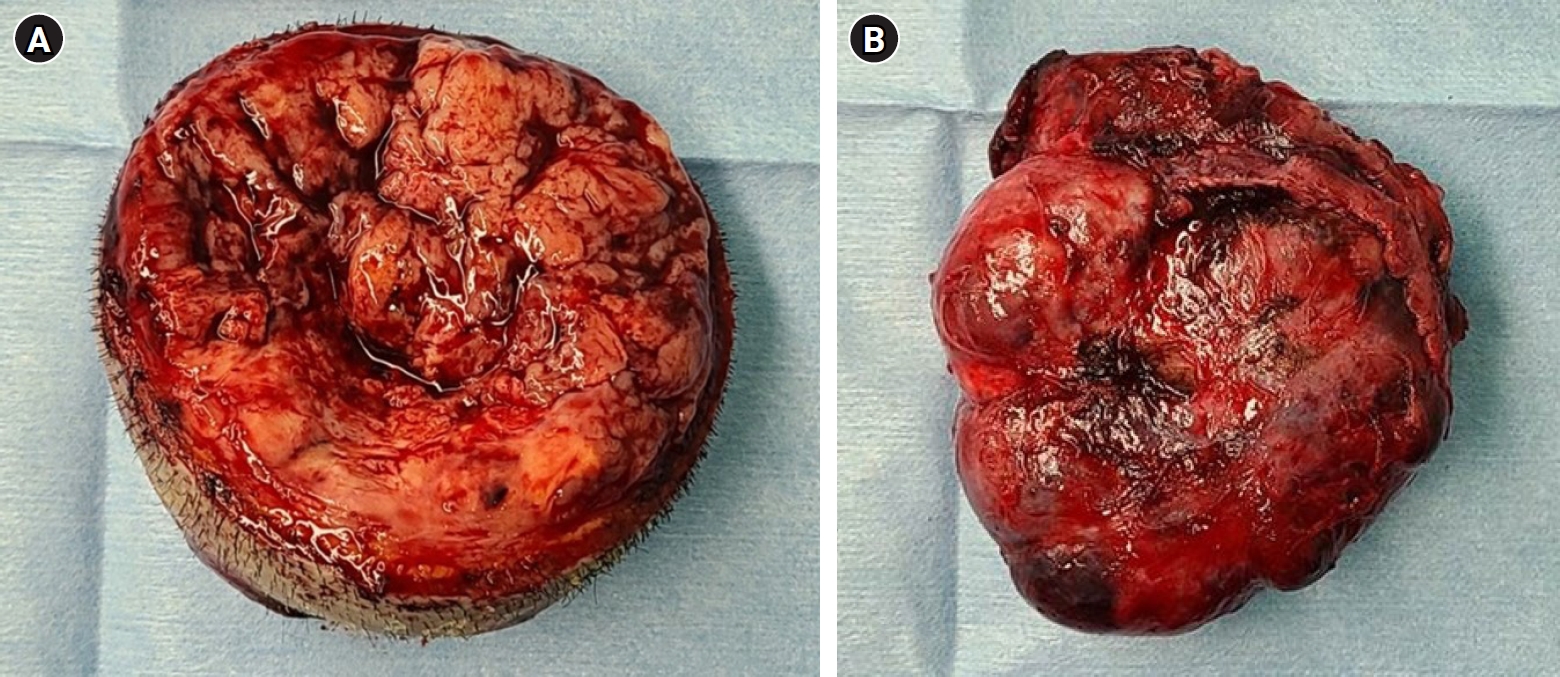
Fig. 4.
A pleomorphic spindle-cell proliferation with branching and hyalinized “staghorn”-shaped blood vessels in variable collagenous stroma (HE stain, x 100) (A). Positive CD34 in immunohistochemical stains (B). A hypocellular appearance and fewer pleomorphic cells in the inferior mass (HE stain, x 400) (C). A hypercellular nature and high mitotic figure (> 10 mitoses/10 HPF) (HE stain, x 400) (D).
References
1. Chung HR, Tam K, Han AY, Obeidin F, Nakasaki M, Chhetri DK, et al. Solitary fibrous tumors of the head and neck: a single-institution study of 52 patients. OTO Open. 2022;6:2473974X221098709.




2. Zhou Y, Chu X, Yi Y, Tong L, Dai Y. Malignant solitary fibrous tumor in retroperitoneum: a case report and literature review. Medicine (Baltimore). 2017;96:e6373.



3. Hwang US, Kim SB, Jo DJ, Kim SM. Intramedullary solitary fibrous tumor of cervicothoracic spinal cord. J Korean Neurosurg Soc. 2014;56:265–8.



4. Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30:1464–73.


5. Chan JK. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology. 1997;31:568–76.



6. Mori S, Lezcano C, Miraflor AP, Busam KJ, Lee EH. Solitary fibrous tumor presenting on the scalp: a potential diagnostic pitfall. J Cutan Pathol. 2018;45:557–60.




7. Kim JH, Kim DC, Lee R, Shin CH, Han YS, Chung SH, Paik SY. Myxoid solitary fibrous tumor on the scalp. Arch Craniofac Surg. 2017;18:269–72.




8. Omori Y, Saeki H, Ito K, Matsuzaki H, Tokita M, Itoh M, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539–41.


9. Rizk T, Awada A, Sebaaly A, Hourani R. Solitary fibrous tumor of the scalp in a child. J Neurosurg Pediatr. 2013;11:79–81.


10. Rabie A, Hasan A, Mohammed Y, Abdelmaksoud A, Rabaan AA. Recurrent malignant solitary fibrous tumor of the scalp: a case report and literature review. J Pathol Transl Med. 2022;56:103–8.




11. Shirley BM, Kang DR, Sakamoto AH. Malignant solitary fibrous tumor of the scalp. J Maxillofac Oral Surg. 2016;15:245–8.




12. Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22:1–31.


13. Raghani N, Raghani MJ, Rao S, Rao S. Hemangiopericytoma/solitary fibrous tumor of the buccal mucosa. Ann Maxillofac Surg. 2018;8:151–3.



14. Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48:3–12.


15. Kwon JH, Song JS, Jung HW, Lee JS, Cho KJ. Malignant solitary fibrous tumor with heterologous rhabdomyosarcomatous differentiation: a case report. J Pathol Transl Med. 2017;51:171–5.




16. Geramizadeh B, Banani A, Moradi A, Hosseini SM, Foroutan H. Intrapulmonary solitary fibrous tumor with bronchial involvement: a rare case report in a child. J Pediatr Surg. 2010;45:249–51.


17. Cox DP, Daniels T, Jordan RC. Solitary fibrous tumor of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:79–84.


18. Tourabi K, Moussaoui A, Khaless A, Achbouk A, Fejjal N, Hjira N, et al. Solitary fibrous tumor of the scalp: a case report. Ann Chir Plast Esthet. 2008;53:526–30.

19. Erdag G, Qureshi HS, Patterson JW, Wick MR. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007;34:844–50.


20. Ramdial PK, Madaree A. Aggressive CD34-positive fibrous scalp lesion of childhood: extrapulmonary solitary fibrous tumor. Pediatr Dev Pathol. 2001;4:267–75.



21. Feasel P, Al-Ibraheemi A, Fritchie K, Zreik RT, Wang WL, Demicco E, et al. Superficial solitary fibrous tumor: a series of 26 cases. Am J Surg Pathol. 2018;42:778–85.


22. Geramizadeh B, Marzban M, Churg A. Role of immunohistochemistry in the diagnosis of solitary fibrous tumor, a review. Iran J Pathol. 2016;11:195–203.


23. Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–5.



24. Chiu CS, Lin CY, Kuo TT, Kuan YZ, Chen MJ, Ho HC, et al. Malignant cutaneous tumors of the scalp: a study of demographic characteristics and histologic distributions of 398 Taiwanese patients. J Am Acad Dermatol. 2007;56:448–52.


25. Ali SZ. Malignant solitary fibrous tumor: cytopathologic findings and differential diagnosis. Cancer Cytopathol. 2010;118:83–9.


26. Reshadi H, Rouhani A, Mohajerzadeh S, Moosa M, Elmi A. Prevalence of malignant soft tissue tumors in extremities: an epidemiological study in syria. Arch Bone Jt Surg. 2014;2:106–10.


27. Pisters PW, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol. 1994;12:1150–5.


28. Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:134–44.


29. Yang XJ, Zheng JW, Ye WM, Wang YA, Zhu HG, Wang LZ, Zhang ZY. Malignant solitary fibrous tumors of the head and neck: a clinicopathological study of nine consecutive patients. Oral Oncol. 2009;45:678–82.


30. Fletcher CDM, Unni K, Mertens F. World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. New York: IARC Press; 2002.
-
METRICS

-
- 0 Crossref
- 858 View
- 14 Download
- Related articles in Keimyung Med J
-
A Case of Malignant Melanoma of the Lacrimal Sac2010 ;29(1)
A Case of Warty Dyskeratoma on Scalp2004 December;23(2)
A Case of Solitary Plasmacytoma of the Femoral shaft1994 ;13(2)
A Case of Primary Malignant Lymphoma of the Breast1989 ;8(1)
One Case of Pregnant Rudimentary Uterus with Rupture1986 ;5(1)