|
 |
- Search
| Keimyung Med J > Volume 42(1); 2023 > Article |
|
Abstract
Subcutaneous panniculitis-like T-cell lymphoma (SPLTCL) which is similar to lobular panniculitis is a subtype of skin lymphoma that is characterized by pleomorphic T cells and benign macrophages. The simultaneous presence of hemophagocytic lymphohistiocytosis (HLH) is the most important and adverse prognostic factor in SPLTCL. SPLTCL is a rare disease with no well-established standard treatment.
We report a child with SPLTCL and HLH, who were successfully treated with the modified NHL (non-Hodgkin lymphoma)-BFM(BerlinŌĆÉFrankfurtŌĆÉM├╝nster)-90 and HLH-2004 protocols. Patient had persistent fever and subcutaneous masses. SPLTCL with HLH was diagnosed by immunohistochemistry, radiology and laboratory results. SPLTCL with HLH has shown high mortality when treated with a combination of intensive anticancer drugs. Thus, we first administered dexamethasone and etoposide. After this, when we used the modified protocol of NHL-BFM-90 and HLH-2004, patient showed complete resolution of the subcutaneous masses and features of HLH, except for persistent hyperferritinemia. We tried etanercept to reduce high serum ferritin with some effects.
In children with diagnosis of SPLTCL with HLH, initiation of immediate and appropriate treatment affects prognosis. Thus, prompt initiation of the agents that can simultaneously control underlying disease as well as secondary HLH could have lead to successful results.
Subcutaneous panniculitis-like T-cell lymphoma (SPLTCL) which is similar to lobular panniculitis is a subtype of skin lymphoma that is characterized by pleomorphic T cells and benign macrophages which infiltrates to subcutaneous tissue. The simultaneous presence of hemophagocytic lymphohistiocytosis is the most important and adverse prognostic factor in SPLTCL [1]. SPLTCL is a rare disease with no well-established standard treatment. Various protocols have therefore been used. Successful treatment of SPLTCL with hemophagocytic lymphohistiocytosis (HLH) in children has rarely been reported [2ŌĆō4].
We present a case of SPLTCL with HLH successfully treated initialy with dexamethasone and etoposide followed by modified NHL (non-Hodgkin lymphoma)-BFM(BerlinŌĆÉFrankfurtŌĆÉM├╝nster)-90 and HLH-2004.
An eight-year-old boy was admitted to the department of pediatric surgery with a two-week history of fever and an abdominal mass. He continued to be febrile with intravenous antibiotics, and because of bicytopenia, he was transferred to the department of pediatrics.
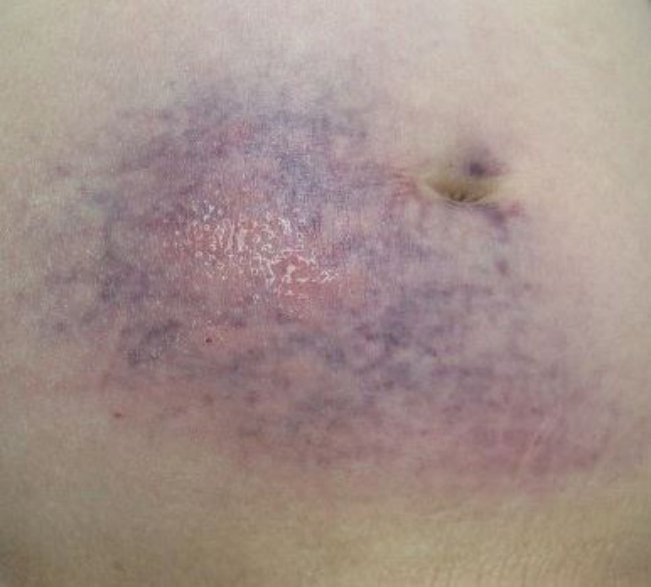
On physical examination, a tender, 15 cm round erythematous swelling on right mid-abdominal wall (Fig. 1) and hepatosplenomegaly (subsequently confirmed by computed tomography) were observed. Initial laboratory findings were: white blood cell count, 2.4├Ś109/dm3; hemoglobin, 99 g/dm3; platelet count, 147├Ś109/dm3; C-reactive protein, 275.24 nmol/dm3 (normal: 0.0ŌĆō47.62 nmol/dm3); triglycerides, 4.16 mmol/dm3 (normal: 0.56ŌĆō1.47 mmol/dm3); fibrinogen, 1.7 g/dm3 (normal: 2.0ŌĆō4.0 g/dm3); soluble interleukin 2 receptor, 14,991 pg/cm3 (normal: 1,398ŌĆō5,513 pg/cm3); ferritin, 1,126.52 pmol/dm3 (normal: 33.71ŌĆō449.44 pmol/dm3) and EpsteinŌĆōBarr virus and cytomegalovirus immunoglobulin titers, negative. Biopsy of the abdominal mass showed subcutaneous infiltration by atypical lymphocytes rimming adipocytes. Immunohistochemistry of the specimen was positive for CD3 and CD8, and negative for CD4, CD20, and CD56 (Fig. 2). T Cell receptor (TCR) gene rearrangement analysis revealed ╔Ż oligoclonality. Whole-body 18F-fluorodeoxyglucose positron emission tomography (PET) revealed increased contrast uptake in subcutaneous tissues of the right abdominal wall, left anterior chest wall, right upper quadrant abdominal wall, left pelvis, bilateral buttocks, and upper legs (Fig. 3). Taken together, these results led to a diagnosis of SPLTCL with HLH.
Treatment with dexamethasone and etoposide was started immediately, and the patient defervesced the next day. After four weeks of HLH induction therapy with dexamethasone and etoposide, NHL-BFM-90 course A was started. When serum ferritin subsequently became elevated above 4,494.38 pmol/dm3, we considered the possibility of HLH aggravation. Thus, four weeks of HLH-2004 induction treatment was repeated with dexamethasone, etoposide, and added cyclosporine A. Thereafter, per protocol, NHL-BFM-90 courses B-A-B-A-B were administered, leading to complete remission with normalized PET results. High ferritin persisted (up to 3,315 pmol/dm3), however. The sustained ferritin elevation was worrisome and suspicious for incomplete control of HLH. A maintenance course of HLH-2004 was therefore continued for a total of 40 weeks. The tumor necrosis factor ╬▒ (TNF- ╬▒) inhibitor etanercept was then administered for a further year. On regular follow-up, the patient has been doing well, except for a ferritin level of approximately 2,247 pmol/dm3.
SPLTCL is a type of skin lymphoma characterized by pleomorphic T cells and benign macrophages that infiltrate to subcutaneous tissue, mimicking lobular panniculitis [3]. It is characterized by cutaneous plaques and nodules which are slowly progressive and have a tendency to ulcerate.
SPLTCL is divided into two subtypes: ╬▒/╬▓ and ╬│/╬┤. In 2005, the World Health Organization (WHO) and European Organization for Research and Treatment of Cancer (EORTC) classification of primary cutaneous lymphomas has restricted the category of SPLTCL to tumors expressing the TCR ╬▒/╬▓, CD4-, CD8+, CD56-. Cutaneous ╬│╬┤ T-cell lymphoma was restricted that expressing the TCR ╬│/╬┤, CD4-, CD8- [1]. This categorization was based on the differences in prognoses between these two, with SPLTCL showing much favorable clinical outcomes [5].
Gayden et al [6] reported in their, recent studies on the cases showing familial predisposition by gene mutation as one of the causes of SPLTCL and HLH. TIM-3, a gene likely related to SPLTCL, is a transmembrane protein expressed by CD8+ T cells and natural killer cells, and a negative immune checkpoint through interactions with cognate ligands included galectin-9 [6,7]. Tyr82Cys and Ile97Met at TIM-3 are the most commonly found mutations in SPLTCL. These two variants induce protein misfolding and membrane expression failure in T cells and monocytes, leading to persistent immune activation and increased production of inflammatory cytokines, promoting SPTCL and HLH [6]. Thus, these authors insisted to consider that immunosuppression and more novel agents targeting IL-1 and IFN-╬│ when the TIM-3 mutation is identified in children with SPLTCL and HLH [6].
The prognosis is poor in malignany-related HLH [8]. Probably the treatment intensity could have been too intensive for induction period. When HLH and malignancy are accompanied, the delay in the diagnosis of HLH due to clinical similarity may also be a contributing factor [9].
Standard therapeutic options for SPLTCL have not been established, and protocols as various as CHOP (cyclophosphamide-vincristine-doxorubicin-prednisolone), SMILE (steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide), NHL-BFM-90, and stem-cell transplantation have been tried [2,4]. CHOP has recently been reported to have little effect. SPLTCL with HLH is a rare disease in children. NHL-BFM-90 was demonstrated to result in relatively good long-term responses with acceptable toxicity in combined SPLTCL and HLH [3,4].
We suspected the present case of SPTCL accompanying HLH. We reviewed the literature of SPLTCL with HLH in children to determine the treatment options for the patient (Table. 1).
Among 11 reported cases, 5 cases were treated with NHL-BFM-90 protocol, 2 cases with CsA and steroid, 2 cases with CHOP and 1 case each with SMILE or CEOP (cyclophosphamide, epirubicin, vincristine, and prednisone). All of NHL-BFM-90 based protocol induced remission. Among 2 cases of patients treated with CHOP, 1 case was induced in remission and another case was expired.
We selected the combination of the NHL-BFM-90 and HLH-2004 protocols. However, when we analyzed the causes of failed cases, most of them expired during initial induction period, indicating that the intensity of the initial treatment might have been too intensive [8].
In addition, treatment of malignant diseases with HLH are known to show no universal conclusions due to lack of large samples or prospective clinical trials [9]. We started dexamethasone and etoposide as these two drugs are included in NHL-BFM-90 and HLH-2004 protocols. We expected these two drugs would work for HLH as well as SPLTCL and not aggravate stormy cytokine reaction. After induction period, when HLH features were improving and subcutaneous masses were decreasing in size, we used anticancer drugs in the NHL-BFM-90 protocol fully.
After complete resolution of the subcutaneous lesions as well as the most features of the HLH were resolved, hyperferritinemia persisted. We had suspicion of incompleted control of HLH related hyperferritinemia [10]. Thus, we tried etanercept which is known to inhibit the activity of TNF- ╬▒ by competitively binding with its cell-surface receptors for further control of HLH [11]. Etanercept has been reported to be useful for controlling inflammatory cytokines in therapy-resistant macrophage activation syndrome [11]. The pathophysiology in secondary HLH and macrophage activation syndrome seems to be similar, with excessive immune activation and hemophagocytosis [11]. Takahashi et al [12] successfully treated lupus-associated HLH with etanercept. Thus, we administered etanercept as an immune system mediator to regulate TNF- ╬▒. Administration of etanercept led to moderately decreased serum ferritin, but it did not produce a response as dramatic as we had expected.
In summary, we report a case in which the modified NHL-BFM-90 and HLH-2004 protocols were applied in a child of SPLTCL with HLH, and successfully treated. We tried etanercept to reduce high serum ferritin, with some effect. Thus, further studies are needed for unresolved hyperferritinemia. In children with diagnosis of SPLTCL with HLH, initiation of immediate treatment affects prognosis. Prompt initiation of agents such as dexamethasone and etoposide that can simultaneously control underlying disease as well as secondary HLH could lead to successful results.
Notes
Fig.┬Ā2.
Histopathologic findings of right mid-abdominal wall nodules. Hematoxylin and eosin stained specimen shows atypical lymphocytes infiltration in the subcutaneous tissue (A). Immunohistostainings are positive for CD3 (B), CD8 (C) and, negative for CD4 (D), CD20 (E), CD56 (F). (A-F, original magnifications: ├Ś200).
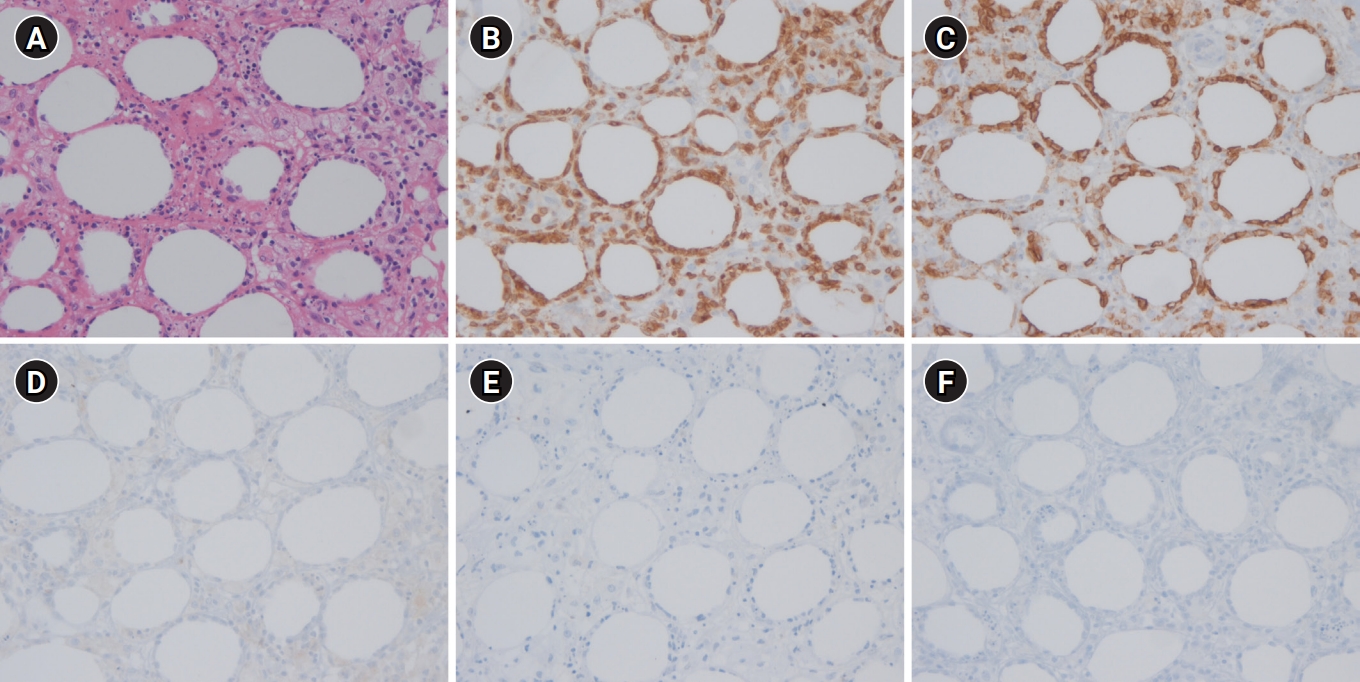
Fig.┬Ā3.
(A) Whole-body positron emission tomography (PET) scan at diagnosis reveals multiple increased fluorodeoxyglucose uptakes in the subcutaneous tissues of right abdominal wall, left anterior chest wall, right upper quadrant abdominal wall, left pelvis, both buttock and upper legs. (B) Follow-up PET scan after the course B-A-B-A-B of the Non-Hodgkin Lymphoma-BerlinŌĆÉFrankfurtŌĆÉM├╝nster-90 protocol shows resolution of most of the lesions.
Table┬Ā1.
Characteristics of pediatric patients with subcutaneous panniculitis-like T-cell lymphoma accompanied by HLH described in the literature
| Age (y), Sex | Country (reference) | Treatment | Outcome | |
|---|---|---|---|---|
| 1 | 14, F | Japan (Sakurai E. et al [3]) | NHL-BFM & ALL-90, auto-PBSCT | In remission at 2y |
| 2 | 11, M | India (Singh S. et al [4]) | NHL-BFM-90 | In remission at 6m |
| 3 | 11, M | Republic of Korea (Kim SJ. et al [13]) | Modifying NHL-BFM-90 & HLH-2004 | In remission at 5y |
| 4 | 11, M | India (Medhi K. et al [14]) | NHL-BFM-90 | In remission at 2y |
| 5 | 5, F | India (Gupta V. et al [15]) | NHL-BFM-90 | In remission at 2y |
| 6 | 13, F | U.S.A (Merritt BY. et al [16]) | Bexarotene+prednisolone, SMILE | In remission at 2y |
| 7 | 7m, F | Singapore (Koh MJ. et al [17]) | CHOP x 7, Allo-SCT | In remission at 1y 10m |
| 8 | 12, M | Singapore (Koh MJ. et al [17]) | CHOP x 2, fludarabine x 4 | Died of sepsis at 15m after diagnosis |
| 9 | 19, M | India (Babu V. et al [18]) | CEOP | in remission at 9m |
| 10 | 19, F | China (Zhou JC. et al [19]) | CsA, Prednisone | Alive at 66m |
| 11 | 17, F | Saudi Arabia (Al Zolibani AA. et al [20]) | CsA, Prednisolone | Alive at 12m |
HLH, hemophagocytic lymphohistiocytosis; NHL, non-Hodgkin lymphoma; BFM, BerlinŌĆÉFrankfurtŌĆÉM├╝nster; PBSCT, peripheral blood stem cell transplantation; SMILE, steroid methotrexate ifosfamide L-asparaginase etoposide; CHOP, cyclophosphamide doxorubicin vincristine prednisone; SCT, stem cell transplantation; CEOP, cyclophosphamide epirubicin vincristine prednisone; CsA, cyclosporine A.
References
1. Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838ŌĆō45.



2. Oschlies I, Simonitsch-Klupp I, Maldyk J, Konovalov D, Abramov D, Myakova N, et al. Subcutaneous panniculitis-like T-cell lymphoma in children: a detailed clinicopathological description of 11 multifocal cases with a high frequency of haemophagocytic syndrome. Br J Dermatol. 2015;172:793ŌĆō7.


3. Sakurai E, Satoh T, Akiko YA, Maesawa C, Tsunoda K, Endo M, et al. Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) with hemophagocytosis (HPS): successful treatment using high-dose chemotherapy (BFM-NHL & ALL-90) and autologous peripheral blood stem cell transplantation. J Clin Exp Hematop. 2013;53:135ŌĆō40.


4. Singh S, Philip CC, John MJ. Pediatric subcutaneous panniculitis-like T-cell lymphoma with hemophagocytosis showing complete resolution with the BFM90 protocol: case report and review of literature. J Pediatr Hematol Oncol. 2019;41:478ŌĆō81.


5. Toro JR, Liewehr DJ, Pabby N, Sorbara L, Raffeld M, Steinberg SM, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407ŌĆō12.



6. Gayden T, Sepulveda FE, Khuong-Quang DA, Pratt J, Valera ET, Garrigue A, et al. Germline HAVCR2 mutations altering TIM-3 characterize subcutaneous panniculitis-like T cell lymphomas with hemophagocytic lymphohistiocytic syndrome. Nat Genet. 2018;50:1650ŌĆō7.



7. Polprasert C, Takeuchi Y, Kakiuchi N, Yoshida K, Assanasen T, Sitthi W, et al. Frequent germline mutations of HAVCR2 in sporadic subcutaneous panniculitis-like T-cell lymphoma. Blood Adv. 2019;3:588ŌĆō95.




8. Rivi├©re S, Galicier L, Coppo P, Marzac C, Aumont C, Lambotte O, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127:1118ŌĆō25.


9. Wang H, Xiong L, Tang W, Zhou Y, Li F. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget. 2017;8:59977ŌĆō85.



10. Stroud J. A mechanistic theory explaining hyperferritinaemia in haemophagocytic lymphohistiocytosis. Med Hypotheses. 2019;122:165ŌĆō71.


11. Sim YS, Kim HS, Kim KN. A case of macropharge activation syndrome successfully treated with combination therapy including etanercept. J Rheum Dis. 2012;19:225ŌĆō9.

12. Takahashi N, Naniwa T, Banno S. Successful use of etanercept in the treatment of acute lupus hemophagocytic syndrome. Mod Rheumatol. 2008;18:72ŌĆō5.


13. Kim S, Kim A, Hah JO. Subcutaneous panniculitis-like T-cell lymphoma with hemophagocytic syndrome in a child: a successful treatment with the BFM-NHL-90 protocol. Clin Pediatr Hematol-Oncol. 2020;27:129ŌĆō33.

14. Medhi K, Kumar R, Rishi A, Kumar L, Bakhshi S. Subcutaneous panniculitislike T-cell lymphoma with hemophagocytosis: complete remission with BFM-90 protocol. J Pediatr Hematol Oncol. 2008;30:558ŌĆō61.


15. Gupta V, Arava S, Bakhshi S, Vashisht KR, Reddy R, Gupta S. Subcutaneous panniculitis-like T-cell lymphoma with hemophagocytic syndrome in a child. Pediatr Dermatol. 2016;33:e72ŌĆō6.


16. Merritt BY, Curry JL, Duvic M, Vega F, Sheehan AM, Curry CV. Pediatric subcutaneous panniculitis-like T-cell lymphoma with features of hemophagocytic syndrome. Pediatr Blood Cancer. 2013;60:1916ŌĆō7.




17. Koh MJ, Sadarangani SP, Chan YC, Chan MY, Tan AM, Tan SH, et al. Aggressive subcutaneous panniculitis-like T-cell lymphoma with hemophagocytosis in two children (subcutaneous panniculitis-like T-cell lymphoma). J Am Acad Dermatol. 2009;61:875ŌĆō81.


18. Babu V, Yadav S, Thorat J, Nayak L, Sengar M, Shet T, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinical features and outcomes from a single tertiary center. Indian J Hematol Blood Transfus. 2021;37:697ŌĆō8.