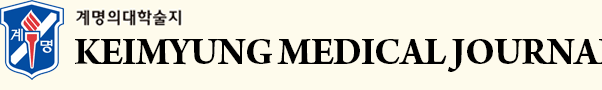|
 |
- Search
| Keimyung Med J > Volume 42(1); 2023 > Article |
|
Abstract
Implantation of a permanent pacemaker is a safe and effective treatment for symptomatic bradycardia. Conventionally, ventricular lead is placed at the right ventricle (RV) muscles. Therefore, this causes interventricular dyssynchrony, and long-term high RV pacing (RVP) burden is associated with an increased risk of heart failure and atrial fibrillation. Hence, attempts to directly pace the cardiac conduction system have been made, and finally, a technique called left bundle branch area pacing (LBBAP) has emerged. In our country, the clinical experience of LBBAP is in the early stages. Especially, LBBAP using standard stylet-driven leads (SDL), a major procedural method performed in our country, is also in the early stages, and there are only a few reports about this method worldwide. Herein, we are reporting our initial experiences of LBBAP with SDL. Compared to conventional RVP performed during the same period, LBBAP required an initial learning period, a more extended procedure, and fluoroscopy time. However, the paced QRS duration was significantly shorter in the LBBAP group (LBBAP group 120.6 ± 13.0 msec, RVP group 165.2 ± 16.0 msec, p < 0.001). It is fascinating that simply adding a ventricular lead delivery sheath can create a whole new outcome, even at centers that are only familiar with the standard tools. Our experience will be helpful in arrhythmia centers that aim to start LBBAP for the first time.
Implantation of a permanent pacemaker (PPM) is a safe and effective treatment for symptomatic bradycardia. However, there are still various problems to overcome, and one of the most critical and challenging problems among them is pacing-induced cardiomyopathy (PICM). This is a phenomenon in which the cardiac function gradually decreases after PPM implantation, particularly in patients with a high ventricular pacing burden (e.g., atrioventricular [AV] block). Conventionally, the ventricular lead is placed at the right ventricular (RV) muscles, where the electromechanical activation of the ventricle starts, leading to myocyte-to-myocyte conduction (conventional RV pacing, RVP). This myocardial conduction results in electromechanical conduction delay, interventricular dyssynchrony, and, consequently, PICM [1,2]. To solve this problem, attempts to pace the cardiac conduction system directly have been made for a long time. A new chapter in clinical conduction system pacing (CSP) began with the report of the first-in-human His bundle pacing study in 2000 [3].
Currently, there are two types of CSP: His bundle pacing (HBP) and left bundle branch area pacing (LBBAP). As its name implies, the HBP aims to pace the His bundle directly; therefore, it is an ideal pacing modality. However, as experience with the procedure accumulates, some limitations have become apparent as follows: technical difficulty with narrow target zones, low implant success in patients with infranodal conduction disease, frequent high pacing thresholds, low sensing amplitudes that results in oversensing issues, and a more significant number of lead revisions due to these problems [4,5]. Hence, LBBAP has been proposed as an alternative to HBP. Although the LBBAP cannot reproduce intrinsic narrow QRS like HBP, it has the advantages of lower pacing thresholds, higher sensing amplitudes, and more stable lead positions. However, this LBBAP was first introduced by Huang et al. in 2017 [6], and therefore, it is still in the early stage, and only medium-scale registry studies have been reported so far [7].
Meanwhile, HBP is performed by using the lumen‐less pacing lead (LLL, SelectSecure 3830 pacing lead; Medtronic Inc., Minneapolis, MN, USA) delivered through a dedicated fixed curve sheath (Medtronic C315 His; Medtronic Inc., Minneapolis, MN, USA) or a deflectable sheath (Medtronic C304; Medtronic Inc., Minneapolis, MN, USA). And most practices with LBBAP also have been performed using a LLL with these sheaths, originally developed for HBP [8]. In Korea, there were limitations in using these tools, so we could not experience LLL-based HBP and LBBAP. Only recently, with proposals by De Pooter et al [8], it became possible to use standard stylet-driven pacing leads (SDLs) delivered through pre-shaped sheaths, which have been introduced in our country. Therefore, the clinical experience of LBBAP in our country is in the very early stages, and there are few official reports about it [9,10]. Herein, we report our initial experiences with LBBAP. This report aims to confirm the feasibility and safety of LBBAP using SDL, even in centers that have not implemented HBP. In addition, we tested whether LBBAP is possible with an anatomical approach and a device programmer without using an electrophysiologic recording system. The recently emerged LBBAP capture subtype issues [7], and our procedural tips will also be discussed.
All patients referred to our center for PPM implantation due to symptomatic bradycardia were enrolled and followed prospectively in the Keimyung University Dongsan Hospital device registry. Since tools for LBBAP have generally been available in South Korea since May of 2021, patients between July 2021 and January 2023 were considered for LBBAP. The LBBAP was primarily considered in patients expected to have a high ventricular pacing burden, and those deemed inappropriate for LBBAP, such as elderly and/or frail patients, were excluded.
To compare the clinical and procedural characteristics, data of patients who underwent conventional RVP within the same study period were collected and analyzed with those of LBBAP patients. Representative chest radiographs and electrocardiography (ECG) of conventional RVP and LBBAP are shown in Fig. 1.
Compared to the conventional PPM procedure, LBBAP requires only one additional tool, a lead delivery sheath for ventricular lead. This is used to provide a robust backup for the lead to penetrate the interventricular muscular septum and was originally designed for HBP or coronary sinus cannulation but is also used for LBBAP. Each manufacturer has its sheath, but currently, only two manufacturers’ sheaths are available in Korea, and one of them is currently in the process of approval by the government. Therefore, our LBBAP procedures were performed with a 5.6 Fr SDL with an extendable helix (Biotronik Solia S60; Biotronik SE & Co KG, Berlin, Germany) delivered through a pre-shaped delivery sheath (Biotronik Selectra 3D; Biotronik SE & Co KG, Berlin, Germany).
As described above, our center’s current method of procedure was derived mainly from that of De Pooter et al [8,11,12]. And after the publication of Gills et al, we adopted continuous monitoring of unipolar pacing characteristics during the lead screwing procedure [13]. All procedures except for ventricular lead implantation were identical to conventional PPM-RVP implantation. Fig. 2 shows step by step approach to LBBAP.
(1) Patient preparation, draping, incision, and pocket creation were the same as conventional procedures. Then, the axillary vein was punctured, and a 0.035 guide wire was inserted.
(2) The delivery sheath was then advanced to the RV over the 0.035 guide wire. Since the sheath has double curves, it is difficult to pass through the tricuspid valve by simply pushing it. Hence, when the sheath tip is about to pass through the TV, it should be advanced with a slight clockwise rotation to enter the RV more easily.
(3) The right anterior oblique view was used to determine the location of the interventricular septum for lead implantation (usually 1 cm inferior and distal to the His bundle region) (Fig. 2A). In the left anterior oblique view, a counterclockwise rotation was applied to the sheath to position it perpendicular to the septum and to ensure a secure backup. Contrast injection was used to confirm whether the sheath tip was in contact with the septal myocardium [14] (Fig. 2B).
(4) Pacing cables were connected to the lead stylet (cathode) and the patient's body or self-retaining retractor (anode) to perform unipolar pacing. The stylet should be fully advanced to the tip of the lead. After that, the pacing lead was introduced, which was inserted until it touched the myocardium. Then, we performed unipolar pacing. If a “W” shape QRS morphology was observed on lead V1, this was evidence that the site was the septal myocardium.
(5) When the location of the lead was judged appropriate for deploying the lead by the operator, it was slightly retracted to extend the helix (Fig. 2C). Then, the lead was advanced until it touched the myocardium by the extended helix (Fig. 2D). At that point, the lead was advanced by rotating it clockwise, penetrating the septal myocardium (Fig. 2E). During lead screwing, continuous unipolar lead stylet pacing was maintained [13].
(6) The lead advancement was stopped when the criteria for successful LBBAP were met (described below). Next, contrast injection was used to confirm the depth of implanted lead tip (Fig. 2F). After checking the lead impedance's normal range, we removed the delivery sheath.
(7) If the LBBAP failed after two lead screwing attempts, we implanted the ventricular lead in the conventional manner (RV apex or RV mid-septal myocardial pacing).
Successful LBBAP was defined as either left bundle branch pacing (LBBP, left-sided conduction system capture) or left ventricular (LV) septal pacing (LVSP, pure myocardial capture). We used the following criteria to confirm the LBBP during the procedure [7,11,14]:
(1) Deep septal lead position confirmed by fluoroscopy, sheath ventriculography, and progressive change of paced QRS morphology with lead rotation.
(2)The appearance of a Qr, qR, rSR, or rSr’ pattern in lead V1
(3)stimulus to peak LV activation time (LVAT, measured as the interval between stimulus and R wave peak time in lead V6 [V6 RWPT]) < 80 ms in patients with baseline narrow QRS or right bundle branch block (RBBB) or < 100 ms in patients with left bundle branch block (LBBB) or interventricular conduction delay. The V6 RWPT should be sustained shortest and constant at both low and high pacing output.
In patients without the r′ in lead V1 despite confirmed deep septal position, narrow-paced QRS, and significant QRS narrowing (compared to right-sided septal pacing), the pacing response was labeled as LVSP.
A recently reported European LBBAP registry study suggested the classification of the LBBP capture subtype [7]. The registry classified the capture location by assessing the LBB/fascicular Purkinje potential to QRS interval and QRS polarity in leads II and III. However, we could not record the LBB/fascicular/Purkinje potentials. Hence, we tried to classify the LBBP capture subtype using the following method: (1) left posterior fascicle pacing (LPFP) was well distinguished because it showed a superior QRS axis; (2) left anterior fascicle pacing (LAFP) showed an inferior QRS axis and left septal fascicle pacing (LSFP) showed an intermediate QRS axis, and the paced QRS morphologies were quite different from the intrinsic QRS in both types while strain pattern ST depression was frequently observed in the limb leads; (3) and in the case of LBBP, the morphology of the intrinsic QRS was almost similar to that of the intrinsic QRS while showing an inferior/intermediate QRS axis.
Continuous variables were expressed as the mean value ± standard deviation or median (interquartile range), which did not follow a normal distribution. Categorical variables were expressed as numbers and percentages. A paired or independent sample t-test was performed to compare the continuous variables. For categorical variables, the chi-square test was used. Statistical analyses were performed using the MedCalc® Statistical Software (version 20.215 MedCalc Software Ltd, Ostend, Belgium). A P-value < 0.05 was considered statistically significant.
During the study period, LBBAP was attempted in 36 patients. Among them, the procedure was successful in 29 patients. The overall success rate was 80.6%. It was demonstrated that LBBAP using SDL was feasible and could be performed safely with only a programmer and anatomical approach, without an electrophysiologic recording system.
The reasons for the failure and the sequential flow leading to a high success rate are as follows. The first patient could not tolerate the prolonged procedure time, and the second patient could not obtain adequate backup with a pre-shaped sheath due to technical or anatomical problems. In the third patient, we succeeded for the first time. In the 4th, 6th, and 17th patients, LBBAP could not be obtained even after two attempts of deep septal pacing. In the 11th patient, a septal perforation occurred, and we have described this experience in detail in the discussion section. The 16th patient, who complained of severe chest pain during the procedure, discontinued LBBAP attempts and switched to conventional RVP. The 16th patient complained of severe chest pain during the procedure, which led to the discontinuation of LBBAP attempts. The patient showed no evidence of myocardial infarction, such as ST-segment elevation, and the pain persisted even after changing the position of the ventricular lead. The pain was relieved after the removal of atrial lead. From the 18th to the 36th patient, we successfully completed the procedure in all cases.
Detailed characteristics of successful LBBAP patients are shown in Table 1. The mean age was 63.6 ± 12.1 years, and 41.4% were male. The LBBAP was attempted in 3 patients with sinoatrial node dysfunction, and the remaining candidates were AV block patients expected to have a high ventricular pacing burden. In addition, we attempted the procedure in patients with normal LV ejection fraction (EF) without structural heart disease. The mean LV EF of our patients was 61.8 ± 9.2%, and most of our patients had narrow baseline QRS or RBBB. One patient had LBBB with a mildly reduced LV EF after mitral valvular surgery and had no coronary vein suitable for cardiac resynchronization therapy (CRT). Another patient had a complete AV block and LV EF of 32% but had no coronary vein suitable for CRT. This patient’s EF was increased to 45% two days after the procedure.
As described above, our procedures were performed with standard SDL delivered through a pre-shaped delivery sheath. Initially, there were three types of sheath curves (length of proximal radius 40 mm, 55 mm, and 65 mm), but later more lengthy types of sheaths were added at each curve (39 cm and 42 cm for each curve), making a total of six types of sheaths currently available in Korea (Supplementary figure 1, Courtesy from Biotronik Korea). Therefore, if the sheath did not provide a suitable backup for lead screwing, it was replaced with a different curve. The total number of sheaths used in the procedure is presented in Table 1. On average, 1.5 sheaths were used.
Compared to RVP patients, the LBBAP group was younger (average age in the LBBAP group 63.6 ± 12.1 vs. RVP group 75.9 ± 9.4, p < 0.001). The lead implantation time (both atrial and ventricular lead insertion time, LBBAP 42.9 ± 12.4 min vs. RVP 27.6 ± 12.6 min, p < 0.001) and total procedure time (time from skin incision to closure, LBBAP 85.5 ± 8.8 min vs. RVP 69.0 ± 12.5 min, p < 0.001) were significantly longer in the LBBAP group. In addition, fluoroscopy time and radiation dose were also higher in the LBBAP group [(fluoroscopy time LBBAP 9.9 ± 3.4 min vs. RVP 5.4 ± 2.4 min, p < 0.001), (Air Kema LBBAP 67.9 ± 45.9 mGy vs. RVP 16.2 ± 13.8 mGy, p < 0.001), (Dose area product LBBAP 10.3 ± 8.7 vs. RVP 3.4 ± 2.4, Gy·cm2, p < 0.001)]. These results are summarized in Table 2.
Individual pacing characteristics of the patient are summarized in Table 1. According to our criteria, LBBP was observed in 28 patients, and LVSP was confirmed in 1 patient. The paced QRS duration was significantly shorter in the LBBAP group (120.6 ± 13.0 msec) than in the RVP group (165.2 ± 16.0 msec) (p < 0.001). In the LBBAP group, paced QRS durations were slightly but significantly prolonged compared to the intrinsic QRS durations (LBBAP 120.6 ± 13.0 msec vs. intrinsic QRS duration 107.3 ± 23.1 msec, p = 0.016). Meanwhile, with LBBAP, the QRS duration shortened from 138.9 ± 18.7 msec to 121.4 ± 11.9 msec (p = 0.051) in patients with baseline QRS duration >110 msec but increased from 93.6 ± 8.9 msec to 120.3 ± 13.8 msec (p < 0.0001) in those with baseline QRS duration < 110 msec.
The mean LVAT of LBBAP, measured by V6 RWPT, was 67.0 ± 12.2 msec. At the time of implantation, the mean bipolar pacing threshold was 0.6 ± 0.1V at 0.4 ms. The mean sensed R wave amplitude was measured at 11.5 ± 3.1 mV in bipolar sensing configuration, and the bipolar impedance measured 694.7 ± 101.1 Ω.
We also experienced capturing the conduction system below the LBB while attempting LBBP. According to our criteria, six patients were classified as LBBP, 12 as LAFP, seven as LPFP, and three as LSFP. In addition, one patient was classified into LVSP, as described above. Representative ECG images of each LBBAP subtype are shown in Fig. 3. The paced QRS durations were significantly different between the groups (LBBP group 107.2 ± 8.3, LAFP group 121.4 ± 13.6, LSFP group 118.3 ± 5.5, LPFP group 129.3 ± 8.0, LVSP 138.0 msec, p = 0.011 with ANOVA).
We switched the procedure to conventional RVP in patients who could not tolerate the procedure or who had significant adverse events during the LBBAP attempt. In patients with successful LBBAP, no significant subacute/delayed complications such as lead revisions, late lead perforations, lead fractures, and any serious adverse cardiac events were observed during the median follow-up duration of 127 days (interquartile range 51-190).
From July 2021 to January 2023, we successfully performed LBBAP on 29 patients. The mean age was 63.6 ± 12.1 years, and 41.4% were male. Three patients had sinoatrial node dysfunction, and the remaining patients were AV block. The total procedure time (time from skin incision to closure) was 85.5 ± 8.8 min, and the mean fluoroscopy time was 9.9 ± 3.4 min. The mean paced QRS duration was 120.6 ± 13.0 msec, and the mean V6 RWPT was 67.0 ± 12.2 msec. The lead parameters immediately after the procedure were as follows and stable for a median follow-up duration of 127 days: mean sensed R wave amplitude 11.5 ± 3.1 mV, mean bipolar pacing threshold 0.6 ± 0.1V at 0.4ms, and the mean bipolar impedance 694.7 ± 101.1 ohm. No serious and life-threatening adverse events have occurred in all attempted patients.
As described above, LBBAP was attempted in 36 patients, and the procedure was successful in 29 patients. The overall success rate was 80.6%, which is lower than recently published studies [7,15]. However, it should be noted that we had no prior experience with LLL for HBP, and we used SDL instead of LLL, unlike other studies. Also, we had to prepare for the procedure with a literature review only and had to perform the LBBAP with 12-lead ECG and a programmer without an electrophysiologic recording system. Despite these limitations, the paced QRS duration of LBBAP and other procedural characteristics were similar to previous study results [7,11].
The learning curve of LBBAP has been reported to be around 50 to 100 cases, and it is known to improve steadily over time [7,16]. In our experience, we successfully performed LBBAP in all patients after the initial 17 cases. However, we did not observe a significant decrease or plateau in procedure/fluoroscopy time. We have presented each case's fluoroscopy and procedure time in a supplementary figure 2.
In LBBAP, unique complications that are not observed in RVP can occur, and this is one of the most critical issues in LBBAP. The representative complications include intraprocedural and/or postprocedural lead dislodgement, lead fracture, and septal perforation. In addition, there is a risk of myocardial infarction caused by septal artery damage, formation of coronary artery/vein fistula, and rising of pacing threshold. The risks of these complications have been reported to be low in recent studies, but they are not negligible. In our experience, one intraoperative septal perforation during lead screwing occurred. This patient had a stress-induced cardiomyopathy accompanying mid to distal septal wall akinesia. Movement of the sheath to the far-left side was observed during the procedure, and we retreated the lead delivery sheath and switched the procedure to RVP. The ventricular lead delivery sheath (Biotronik Selectra 3D; Biotronik SE & Co KG, Berlin, Germany) has an outer diameter of 8.7Fr (2.91 mm), which can cause iatrogenic ventricular septal defects (VSD) of about 3mm. However, the follow-up echocardiography of our patient did not reveal any VSD. It appears that the iatrogenic VSD of around 3 mm in size can spontaneously close, and a recent study has not observed the occurrence of iatrogenic VSD as a chronic complication [7].
Capturing the proximal LBB would be ideal, but it is often difficult due to the anatomical variation between individuals, the technical limitations we have so far, as well as the limited procedure time. Since the left conduction system is extensively panned in the LV endocardium [17], reaching any of these would result in much faster LV activation than myocardial pacing. Also, targeting the left fascicle/Purkinje potential would be much easier because the target zone is incomparably wider than proximal LBB. Therefore, additional research is needed to determine whether left anterior/septal/posterior fascicular pacing has no difference compared to HBP/LBBP in terms of LV synchrony and long-term clinical outcome.
For LBBAP, the pacing lead needs to penetrate the interventricular septum, but it cannot be sufficiently supported by the stylet alone. Therefore, stable backup support from the sheath is required for the lead to penetrating the septum. Hence, if it is determined that the sheath is not providing sound backup, we strongly recommend changing to a sheath with a different curve/length. We offer the following three criteria for determining whether a sheath is suitable for the LBBAP procedure:
1) The sheath should be able to reach the LBBAP target zone. If the curve and length of the sheath are too short, it may be difficult to cross the TV, or if it does, it may be positioned at the very proximal septum. This would favor HBP rather than LBBAP.
2) When sufficient counterclockwise rotation power is applied, the sheath should not be slipped off. After confirming that the sheath is in good contact with the septum, a sufficient counterclockwise rotation power is given to the sheath for the test. If the sheath is not slipped and maintains a stable position on the septum, it can be considered a suitable sheath and site for lead screwing. If the sheath slipped off, this is a sign that the sheath needs to be replaced (sheath slip-off sign).
3) During the implantation of the lead into the septum, the sheath should not slide backward, causing the lead to bend upward. If the sheath slides backward during lead screwing, it means that the sheath does not provide adequate backup, which is also a sign that the sheath needs to be replaced (sheath slide-backward sign). Fig. 4 provides illustrative images of these two signs.
While LBBAP is a way to fundamentally solve PICM that has not been overcome for a long time, problems still should be resolved. Although prolongation in the procedure time and fluoroscopy time and the increase in complications are inevitable with any new procedure, such shortcomings tend to decrease as experience accumulates. In addition, short-term and mid-term results are continuously being published and seem promising, but long-term and randomized study results are still lacking. Finally, since the SDL was originally developed and tested for RVP and the LLL was originally developed for HBP, more dedicated leads and tools for LBBAP should be developed and evaluated.
In this report, we described our center’s initial experiences of LBBAP. We had no experience with HBP and HBP using LLL but successfully started LBBAP with SDL. It was encouraging that by adding only a ventricular lead delivery sheath to the tools that were familiar to us, a procedure that resulted in a completely different clinical outcome compared to the conventional RVP could be achieved [18]. Although prolongation of the procedure time and increase in radiation dose and initial learning period were observed, the paced QRS duration was significantly shortened compared to RVP. As always, if the long term-clinical outcomes of the patients can be changed, the hurdles of a new procedure are well worth overcoming.
Supplementary materials
Supplementary materials can be found via https://doi.org/10.46308/kmj.2023.00052.
Notes
Fig. 1.
Representative chest radiograph and electrocardiography (ECG) of conventional right ventricular myocardial pacing (RVP) (A-C) and left bundle branch area pacing (LBBAP) (D-F). In RVP, the ventricular lead was placed at the RV upper septum, and the ECG showed a wide-paced QRS duration. In LBBAP, the ventricular lead was placed at the deeper interventricular septum, and the ECG showed a near-normal QRS duration with RBBB morphology. (A) posteroanterior (PA) chest radiograph of RVP, (B) left-lateral (LL) chest radiograph of RVP, (C) ECG of RVP, (D) PA chest radiograph of LBBAP, (E) LL chest radiograph of LBBAP, (F) ECG of LBBAP.

Fig. 2.
A stepwise approach of ventricular lead insertion for left bundle branch area pacing. Detailed explanations of Figure (A~F) were described in the methods section of the manuscript. Fluoroscopy image of the final position of the atrial and ventricular leads in (G) right anterior oblique and (H) left anterior oblique view.

Fig. 3.
Representative electrocardiography of each left bundle branch area pacing subtypes. Baseline and paced QRS morphology of left bundle branch pacing (LBBP) (A, B), left anterior fascicle pacing (LAFP) (C, D), left septal fascicle pacing (LSFP) (E, F), left posterior fascicle pacing (LPFP) (G, H), left ventricular septal pacing (LVSP) (I, J).
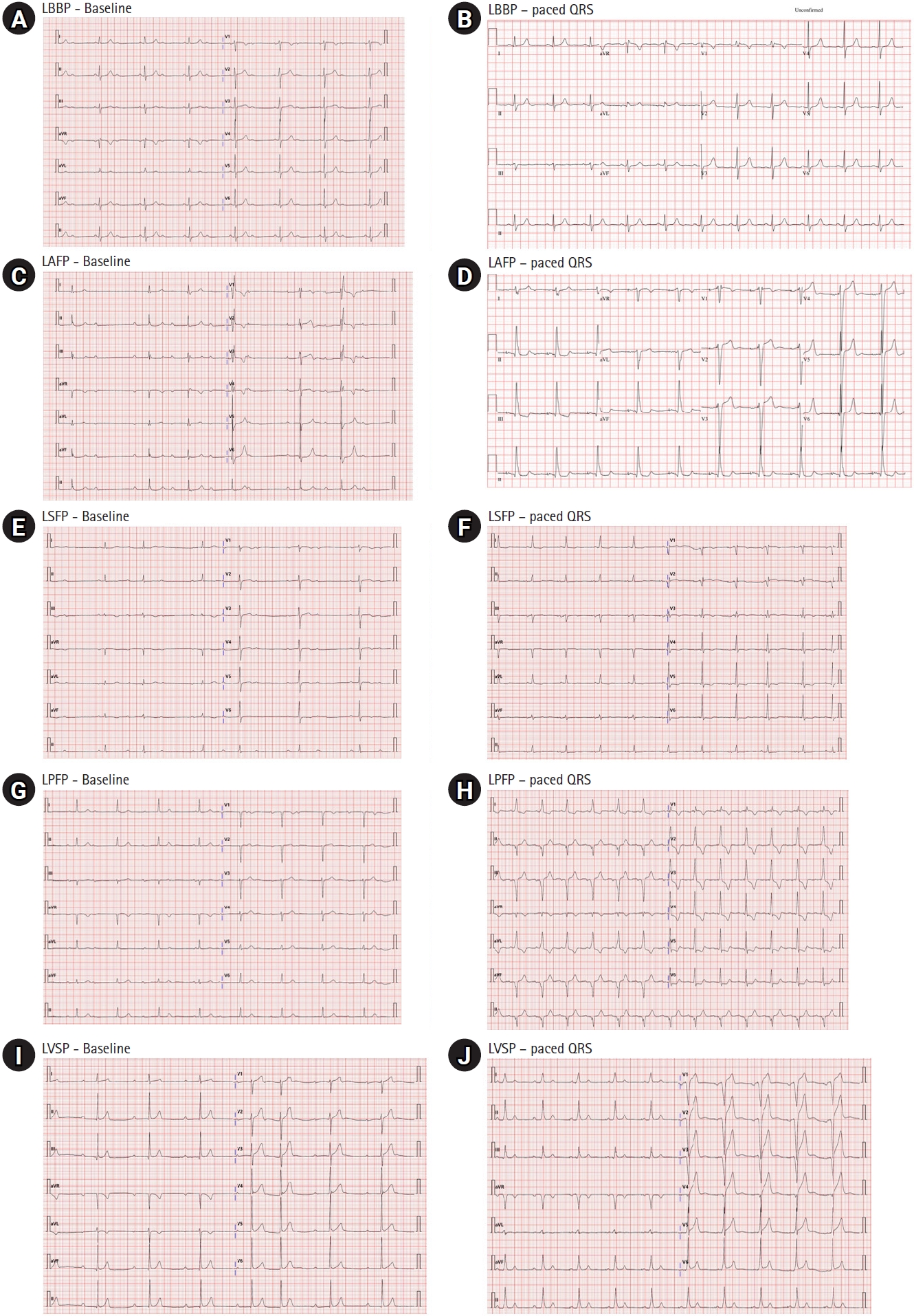
Fig. 4.
Fluoroscopic images of two signs indicate the need to replace the sheath. (A, B) The sheath slip-off sign. After confirming that the sheath is in the LBBAP target zone and has good contact with the septum, a sufficient counterclockwise rotation power was given to the sheath for the test (A). However, the sheath slipped off from the septum (B, white arrow), which is a sign that the sheath needs to be replaced. (C, D) The sheath slide-backward sign. In the position of (C), the lead was advanced with a clockwise rotation for LBBAP. However, the sheath slid backward, near the ring electrode (D, white arrow), causing the lead to bend upward. It means that the sheath does not provide adequate backup, which also indicates that it needs to be replaced.
Table 1.
Characteristics of patients with successful left bundle branch area pacing
Values are presented as the n (%) or mean ± SD. LVEF, left ventricular ejection fraction; V6 RWPT, interval between stimulus and R wave peak time in lead V6; 2AVB, second degree atrioventricular block; SAND, sinoatrial node dysfunction; 3AVB, third degree (complete) atrioventricular block; IVCD, interventricular conduction delay; RBBB, right bundle branch block; LBBB, left bundle branch block.
*Pulse width was setted at 0.4ms
Table 2.
Comparing demographic and procedural characteristics between two groups
References
2. Merchant FM, Mittal S. Pacing induced cardiomyopathy. J Cardiovasc Electrophysiol. 2020;31:286–92.



3. Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101:869–77.


4. Teigeler T, Kolominsky J, Vo C, Shepard RK, Kalahasty G, Kron J, et al. Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Heart Rhythm. 2021;18:743–9.


5. Zanon F, Abdelrahman M, Marcantoni L, Naperkowski A, Subzposh FA, Pastore G, et al. Long term performance and safety of His bundle pacing: a multicenter experience. J Cardiovasc Electrophysiol. 2019;30:1594–601.



6. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736, e1–3.


7. Jastrzebski M, Kielbasa G, Cano O, Curila K, Heckman L, De Pooter J, et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43:4161–73.




8. De Pooter J, Calle S, Timmermans F, Van Heuverswyn F. Left bundle branch area pacing using stylet-driven pacing leads with a new delivery sheath: a comparison with lumen-less leads. J Cardiovasc Electrophysiol. 2021;32:439–48.



9. Byeon K, Kim HR, Park SJ, Park YJ, Choi JH, Kim JY, et al. Initial experience with left bundle branch area pacing with conventional stylet-driven extendable screw-in leads and new pre-shaped delivery sheaths. J Clin Med. 2022;11:2483.



10. Kim T-H, Yu G-I, Yu HT, Joung B, Pak H-N, Lee M-H. Learning curve and initial experience for left bundle branch area pacing with standard stylet-driven pacing leads: comparison with conventional right ventricular pacing. Authorea; 2022. DOI: 10.22541/au.166184287.77298528/v1.
11. De Pooter J, Ozpak E, Calle S, Peytchev P, Heggermont W, Marchandise S, et al. Initial experience of left bundle branch area pacing using stylet-driven pacing leads: a multicenter study. J Cardiovasc Electrophysiol. 2022;33:1540–9.



12. De Pooter J, Wauters A, Van Heuverswyn F, Le Polain de Waroux JB. A guide to left bundle branch area pacing using stylet-driven pacing leads. Front Cardiovasc Med. 2022;9:844152.



13. Gillis K, O'Neill L, Wielandts JY, Hilfiker G, Vlase A, Knecht S, et al. Left bundle branch area pacing guided by continuous uninterrupted monitoring of unipolar pacing characteristics. J Cardiovasc Electrophysiol. 2022;33:299–307.



14. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–6.


15. Padala SK, Master VM, Terricabras M, Chiocchini A, Garg A, Kron J, et al. Initial experience, safety, and feasibility of left bundle branch area pacing: a multicenter prospective study. JACC Clin Electrophysiol. 2020;6:1773–82.


16. Wang Z, Zhu H, Li X, Yao Y, Liu Z, Fan X. Comparison of procedure and fluoroscopy time between left bundle branch area pacing and right ventricular pacing for bradycardia: the learning curve for the novel pacing strategy. Front Cardiovasc Med. 2021;8:695531.



17. Tawara S. Das reizleitungssystem des säugetierherzens - eine anatomisch - pathlogische studie über das atrioventrikularbündel und die purkinjeschen fäden. Jena: Verlag von Gustav Fischer; 1906.