Introduction
Glioblastoma multiforme (GBM) is the most lethal type in primary brain tumors, with most patients dying within one year after initial diagnosis. Its treatment as surgery with radiotherapy and chemotherapy has induced 2 and 5 years survival rates of 25 and 10%, respectively [1]. Moreover, clinical trials have validated only limited benefits of targeted regimens, because of invasive nature and low proliferative activity of GBM [2].
Transcription factors have chief roles in the regulation of cell development and maintenance; they are essential for cell maintenance, proliferation and apoptosis [3]. In these factors, GA-binding protein A forms a heterotetramer complex with its partner GA Binding Protein Transcription Factor Subunit Beta (GABPB) 1 or GABPB2; GABPB1 gene encodes the GABPB1 with its isoforms GABPB1-longer (GABPB1L) and GABPB1-shorter as the products of a different mRNA splicing. Similar to many other E-twenty-six (ETS) factors, they also had oncogenic activities and were associated with the pathogenesis of leukemia, prostate cancer, and other malignancies [4-6]. Recent study introduced novel role of GABPB1L and telomerase reverse transcriptase (TERT) in GBM [7]. When combined with temozolomide chemotherapy, inducible GABPB1L knockdown and the associated TERT reduction led to an impaired DNA damage response that resulted in profoundly reduced growth of GBM tumor in vivo. Our previous study also demonstrated that GBM with TERT mutation had self-renewal capacity inducing longer telomere and poorer survival result [2]. However, this hypothesis was not observed in cancers using big data.
Here, we analyzed prognostic value of TERT and GABPB1L mRNA expression in GBM, based on the Cancer Genome Atlas (TCGA) data. And the correlation between GABPB1L and TERT mRNA expressions was also investigated.
Materials and methods
The expression level of TERT and GABPB1L in GBM was assessed using TCGAŌĆÖs data portal (https://tcga-data.nci.nih.gov/tcga/) on July, 2021. TCGA is a public online database consisting of publicly available microarray data. The RNA-seq gene expression dataset and the clinicopathological parameters for the TCGA GBM were downloaded from the UCSC Xena Browser (https://xena.ucsc.edu/, date last accessed on July 2021). The evaluated GBM of RNA-seq dataset was included 152 samples. Gene expression was measured by Affymetrix HT Human Genome U133a microarray platform and its expression level was calculated as log2(x). This study met the publication guidelines provided by TCGA (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga/using-tcga/citing-tcga, date last accessed on 31 July 2021).
The patients were divided into higher and lower groups by cut-off value for the median expression of each gene. And its clinicopathological values were analyzed by chi-squared test for categorical variables. Spearman's correlation analysis was analyzed the correlation between TERT and GABPB1L. Survival analysis was estimated using the Kaplan-Meier univariate analysis by the log-rank test. Overall survival was defined as the time between diagnosis and mortality and disease free survival was defined as the time between diagnosis and disease recurrence. This statistical analysis was performed by SPSS version 26.0 for Windows (SPSS Inc, Chicago, IL, USA) and p-Value less than 0.05 was considered statistically significant.
Result
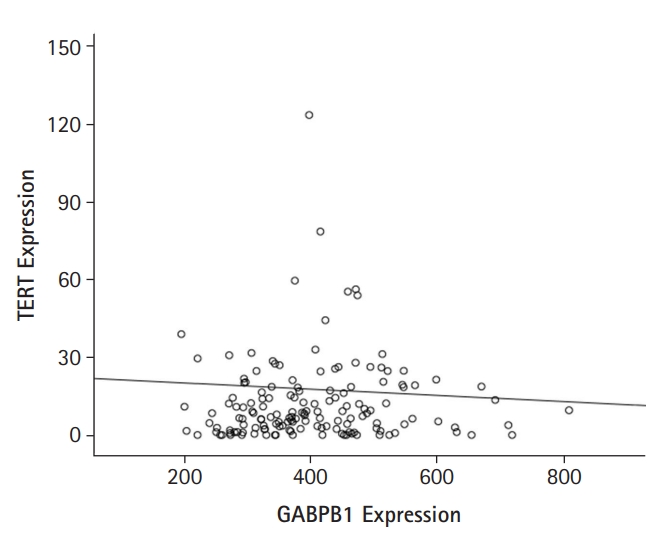
Expression data were downloaded from TCGAŌĆÖs data portal (https://tcga-data.nci.nih.gov/tcga/) on July, 2021. TCGA data indicated that GABPB1L mRNA expression levels did not correlate with those of TERT mRNA (r = -0.027, p = 0.744, Fig. 1). Then, clinical characteristics of GABPB1L and TERT expressions in GBM were summarized in Table 1. Their expression was associated with subtype by gene expression in TCGA data.
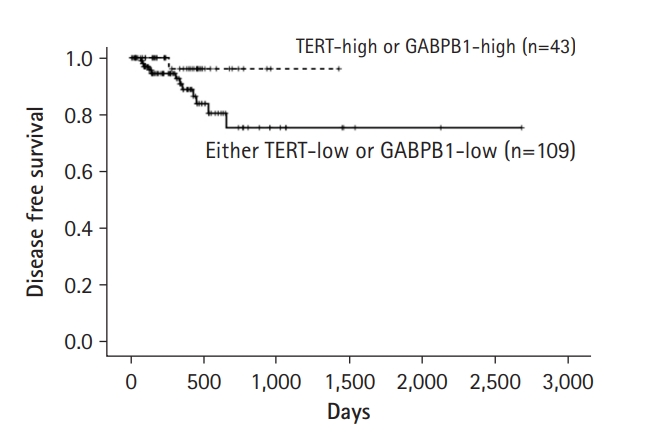
Furthermore, the prognostic value of GABPB1L and TERT mRNA expression was analyzed in GBM the basis of TCGA data. GABPB1L (562.7 ┬▒ 76.8 days vs. 479.1 ┬▒ 5.8 days, p = 0.401) and TERT (468.1 ┬▒ 43.6 days vs. 565.3 ┬▒ 75.8 days, p = 0.403) did not have prognostic value. Then, we classified GBM patients into four groups according to GABPB1L and TERT expression. The overall survival rate was not different in these groups. However, disease-free survival was tended to be different in GBM patients (Fig. 2). GABPB1L-high and TERT-high groups showed better survival results compared to other groups though it did not get statistical significance (2122.6 ┬▒ 160.2 days vs. 1381.1 ┬▒ 244.0 days, p = 0.072).
Discussion
Genetic studies in GBM patients have revealed various genetic alterations, including isocitrate dehydrogenase gene mutations and mutations in the promoter region of telomerase reverse transcriptase gene [8,9]. TERT promoter mutations produced novel binding motifs for E26 transformation-specific/T-cell factor transcription factors, and increased transcriptional activity inducing telomere elongation [2,10]. Previous studies showed that TERT mutation generated a purine-rich GGAAG binding site for the transcription factor GA-binding protein of the ETS family and GABPB1L activated TERT promoter mutation [7,10,11]. It indicated these genetic changes were associated each other and it contributed to GBM progression. For a first time, we analyzed the correlation between GABPB1L and TERT mRNA expression in GBM and their clinicopathological and prognostic values were investigated.
Unlike previous study [7], we found a no association between TERT and GABPB1 expression. Recent study reported negative correlation between TERT and GABPB1 expression in grade III gliomas [12]. Interestingly, clinicopathological parameter of subtype showed a similar pattern with TERT (p = 0.007) and GABPB1L (p = 0.031) expression levels. Previous studies demonstrated that TERT expression induced poorer prognosis via cancer progression [1,2,5]. However, big data demonstrated that both TERT and GABPB1L expression did not have any prognostic values. Moreover, according to our stratified classification by TERT and GABPB1L expression, their higher expressions showed a better prognostic value in disease free survival. Though TCGA data did not include the information such as chemotherapy, big data from GBM patient tissues may be different with cell line study [7]. Therefore, the paradoxical effect of TERT and GABPB1L in cancer study should be confirmed further. The biological functions of TERT and GABPB1 regulation are of great interest for further studies in various cancers.
The present study revealed that TERT and GABPB1L may have clinical values in GBM patients. We suggested that TERT and GABPB1L may represent valuable biomarkers to predict GBM prognosis. Further investigations are warranted to achieve a better understanding of their function in GBM.