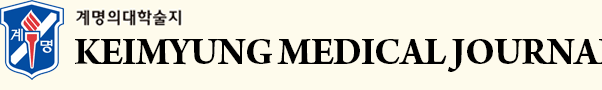|
 |
- Search
| Keimyung Med J > Volume 39(2); 2020 > Article |
|
Abstract
Severe skin reactions complicating tumor necrosis factor-alpha (TNF-α) antagonist therapy were somewhat reported in adults. We report a case of 24-month-old boy with refractory Kawasaki disease who developed toxic epidermal necrolysis (TEN) after one dose of 5mg/kg infliximab. The patient responded to intravenous hydrocortisone and was able to discharge without any other serious complications. The clinicians should be aware of the potential for severe skin reactions from the use of TNF-α antagonists in children.
Recently, infliximab (Remicade®), a monoclonal antibody that blocks the biological activity of tumor necrosis factor-alpha (TNF-α), was used in many cases of refractory Kawasaki disease (KD) patients and it was reported to be effective [1,2]. But, TNF-α antagonists had several adverse reactions, such as serious infections, optic neuritis, aplastic anemia, congestive heart failure and multiple cutaneous adverse effects such as eczema, erythema urticaria, lupus-like syndrome and even Stevens-Johnson syndrome (SJS)/Toxic epidermal necrolysis (TEN) [3]. A few numbers of cases have been reported of SJS induced by anti-TNF-α therapy in adults with Crohn’s disease and rheumatoid disease [3-5], nevertheless, there was no reported similar cases in children. Herein, we describe a case of TEN induced by infliximab therapy in refractory KD.
A 2-year-old boy admitted as KD in our hospital. He had a fever for 5 days, pleomorphic rash, lip redness, conjunctival injection, and left cervical lymph node enlargement. Despite treatment with intravenous immunoglobulin (IVIG, 2g/kg) and corticosteroid pulse therapy (methylprednisolone 30mg/kg/day for 3 days), he remained febrile, and a transthoracic echocardiogram revealed coronary arteries dilatation [LM=3.1-3.3mm (z-score:2.08-2.6), LAD=2.4mm (z-score:2.28), RCA=2.4-3.0mm (z-score:1.83-3.64)]. So, he was treated with infliximab 5mg/kg, and the patient’s fever subsided. All clinical symptoms of KD subsided within 5 days and he was discharged from the hospital.
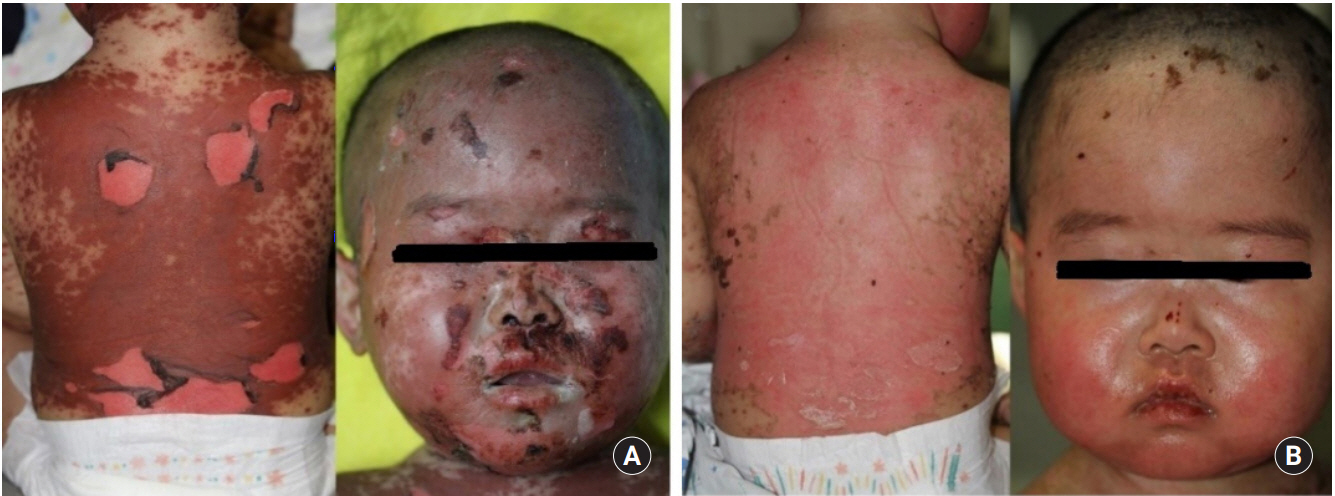
After 10 days from discharge, he developed fever, skin redness, swelling, blistering and skin abrasions. The skin lesions started from the face, back and chest and went worse in the first 7 days, involving more than 30% body surface area, consistent with TEN (Fig. 1). He was treated with intravenous corticosteroid (prednisolone 2mg/kg/day for 4 days and tapered within 2 weeks) and topical dressings with topical steroids, mupirocin oint and topical moisturizer for 3 weeks. After that, the skin lesions recovered gradually, and there were no respiratory complications. The tests for bacterial and viral serologies, tuberculosis, and allergic tests were all negative.
Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine implicated in the pathogenesis of various autoimmune and rheumatic diseases. TNF-α antagonist is presently approved for use in adults with inflammatory bowel disease (Crohn’s disease, ulcerative colitis), ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, and psoriasis [6]. Although in pediatric patients, infliximab is not presently approved for use in KD; it has been used for IVIG-resistant KD from 2004, and it’s effectiveness was proved in many studies [1].
TNF-α antagonists had several adverse reactions such as serious bacterial infections, tuberculosis, optic neuritis, aplastic anemia, congestive heart failure and multiple cutaneous adverse effects such as eczema, erythema urticaria, lupus-like syndrome, SJS, and TEN [3,7-9]. Especially in pediatric group, several neuropsychiatric disorders, thrombocytopenia, and recurrent viral infection were reported [10]. With the increasing number of patients receiving an anti-TNF-α agent, less common adverse reactions can occur. SJS and TEN are rare and severe cutaneous adverse reactions, characterized by the triad of mucous membrane erosions, target lesions and epidermal necrosis with skin detachment [11]. SJS and TEN are rare with an estimated incidence of 1.5 cases per million per year, but can be fatal with the mortality rate of up to 30-50% [11].
There were several cases of severe skin reactions complicating TNF-α antagonist therapy in adults [3-5]. These cases were treated with etanercept or adalimumab for Crohn’s disease or rheumatoid disease, and they were recovered after steroid treatment with discontinuation of the causative drug. There was no similar report of severe skin reactions caused by TNF-α antagonists in children. Conversely, there were several pediatric cases of TEN or SJS treated with infliximab [12,13]. We reported the pediatric case of TEN developed after use of infliximab (5mg/kg) treatment for refractory KD. And he recovered after intravenous hydrocortisone without any other serious complications.
In conclusion, given that the incidence of infliximab therapy for refractory KD in children, the clinicians should be aware of the potential for severe skin reactions from the use of TNF-α antagonist.
Notes
Ethics approval
This study was approved by the institutional review board of the Keimyung University Dongsan Medical Center (approval number: 2019-12-013).
References
1. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e99.


2. Hur G, Song MS, Sohn S, Lee HD, Kim GB, Cho HJ, et al. Infliximab treatment for intravenous immunoglobulin-resistant kawasaki disease: a multicenter study in Korea. Korean Circ J. 2019;49:183–91.


3. Mounach A, Rezqi A, Nouijai A, Ghozlani I, Achemlal L, Maghraoui AE, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351–3.



4. Salama M, Lawrance IC. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn's disease. World J Gastroenterol. 2009;15:4449–52.



5. Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, Placek W. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14–6.



6. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–79.


7. Lindhaus C, Tittelbach J, Elsner P. Cutaneous side effects of TNF-alpha inhibitors. J Dtsch Dermatol Ges. 2017;15:281–8.

8. Miki H, Okamoto A, Ishigaki K, Sasaki O, Sumitomo S, Fujio K, et al. Cardiopulmonary arrest after severe anaphylactic reaction to second infusion of infliximab in a patient with ankylosing spondylitis. J Rheumatol. 2011;38:1220.


9. Patel D, Madani S, Patel S, Guglani L. Review of pulmonary adverse effects of infliximab therapy in Crohn's disease. Expert Opin Drug Saf. 2016;15:769–75.


10. Gerloni V, Pontikaki I, Gattinara M, Fantini F. Focus on adverse events of tumour necrosis factor alpha blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum Dis. 2008;67:1145–52.


11. Koh MJ, Tay YK. An update on Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Curr Opin Pediatr. 2009;21:505–10.