Tumor Size is Associated with Long-Term Outcomes after Resection of Gastric Gastrointestinal Stromal Tumors
Article information
Abstract
The clinical outcomes after surgical resection of gastrointestinal stromal tumors (GISTs) vary widely due to the differences in tumor size and mitotic index. To analyze the long-term outcomes and prognosis of surgically resected gastric GISTs according to tumor size. We retrospectively analyzed the medical records of 269 patients who underwent surgery for GISTs at Keimyung University Dongsan Hospital from March 2000 to March 2017. We surveyed tumor size, mitotic index, recurrence after surgery, time to recurrence, treatment for recurrence, and mortality. The risk of recurrence of gastric GISTs was classified as very low, low, intermediate, and high risk according to the 2007 Journal of the national comprehensive cancer network (JNCCN). After excluding 69 patients who had simultaneous gastric adenocarcinoma, the outcomes of 200 patients were analyzed. Recurrence was observed in 7 patients: 1 in the very low risk group (1-2 cm), 2 in the very low risk group (less than 5 cm), and 3 in the high risk group. Death due to gastrointestinal bleeding occurred in 1 patient in the high risk group who had a tumor >10 cm. While the recurrence rates after surgical resection of GIST are very low, careful monitoring and regular follow-up are warranted, even for low risk patients.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common tumors of mesodermal origin in the gastrointestinal (GI) tract [1]. GISTs originate from the interstitial cells of Cajal in the muscular layer of the GI tract. Activating mutations in oncogenes such as KIT and PDGFRA are known to be associated with the development of GISTs [2,3]. GISTs account for less than 1% of all malignant tumors in the GI tract, and while they occur most commonly in the stomach (70%), they can occur anywhere in the GI tract, including the small intestine (20–30%) or the rectum/colon (10%) [4]. The standard treatment for GISTs, including gastric tumors, is surgical resection. The aim of surgery is complete resection to ensure that no tumor is left in the resection margin and to avoid rupture of the tumor, which can increase the risk of recurrence [5]. However, the clinical course after surgical resection varies greatly. While GISTs can have a very slow, benign course, they often progress very rapidly even after meticulous surgical resection [6,7]. This is because the prognosis for gastric GISTs is affected by tumor size and mitotic index [8,9]. To date, there have been almost no reports on the long-term prognosis, including recurrence, after surgical resection of gastric GISTs. Therefore, in this study, we aimed to analyze the long-term therapeutic outcomes and prognoses of surgically resected gastric GISTs according to tumor size.
Materials and Methods
Patients
This was a single center study of patients who had undergone surgery at a tertiary medical institution in Daegu. We retrospectively analyzed the medical records of 269 patients who underwent surgery for gastric GIST at Keimyung University Dongsan Medical Center between March 2000 and March 2017. We collected data on the patients’ age and sex, the location of the tumor and its pathological characteristics, and recurrence rates. None of the patients showed remote metastasis at the time of the initial diagnosis. This study was reviewed and approved by Institutional Review Board and Ethics Committee of Keimyung University Dongsan Hospital.
Pathological analysis
For pathological characteristics, we examined tumor size, mitotic index, extent of infiltration in the surgical margin, tumor necrosis, and invasion of nearby lymph nodes. Tumor size was measured as the length of the longest diameter, and mitotic index was calculated in a 50x high power field (HPF). In all patients, gastric GIST was diagnosed using CD117 (c-kit) and CD34 immunostaining. Based on the 2007 JNCCN criteria, the risk of postoperative metastasis was evaluated using tumor size and mitotic index, and patients were classified into very low risk, low risk, intermediate risk, and high risk groups. The very low risk group was defined as a tumor size of <2cm and mitotic index of <5/50HPF; the low risk group was defined as a tumor size of 2–5cm and a mitotic index of <5/50HPF; the intermediate risk group was defined as a tumor size of <5cm and a mitotic index of 6–10/50HPF; and the high risk group was defined as a mitotic index of >10/50HPF, irrespective of tumor size.
Postoperative monitoring
All patients were monitored postoperatively to check for recurrence. The mean follow-up period was 67 months (0.2–200 months). At the outpatient visits during this period, the patients’ medical history was taken and they underwent a physical examination. Upper GI endoscopy and abdominal computed tomography (CT) were performed at 3–6 month intervals, and abdominal ultrasonography, thoracic CT, and positron emission tomography-CT were performed as necessary. Recurrence was defined as reappearance of the tumor on a single test.
Statistical methods
The patients’ demographic characteristics and clinical and pathological data are presented using descriptive statistics. For continuous variables, the number of patients (%), the mean ± standard deviation, and the mean (range) is presented. For categorical variables, the frequency and percentage are presented. The Kaplan-Meier method was used to calculate recurrence-free survival, while the log-rank method was used to determine the factors associated with recurrence. The tests performed were two-tailed and P <0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows ver. 22.0 (IBM Co., Armonk, NY, USA).
Results
Patients’ demographic characteristics
A total of 269 patients underwent surgery for gastric GIST, of whom 69 were excluded because they had simultaneous gastric adenocarcinoma, which would have confounded the analysis of recurrence rates. There were 200 patients in the final analysis. Based on their pathological results, 38 patients were classified in the very low risk group, 98 in the low risk group, 45 in the intermediate risk group, and 19 in the high risk group (Fig. 1). The patients’ mean age was 60.8 ± 11.7 years, and there were was a predominance of females (127 patients). There were 6 patients with a tumor size <1 cm (3%), 37 with a tumor size of 1–2 cm (18.5%), 104 with a tumor size of 2–5 cm (52%), and 53 with a tumor size of >5cm (26.5%). The mean follow-up duration was 67 months (0.2–200 months) (Table 1).

Recurrence risk classification
The very low, low, intermediate, and high risk groups were classified based on the 2007 JNCCN. Sixty nine patients who had simultaneous gastric adenocarcinoma were excluded.
Risk of recurrence according to tumor size
The 6 patients with tumors <1 cm were all in the very low risk group. Of the 37 patients with a tumor size of 1–2 cm, 32 (86.5%) were in the very low risk group and 5 (13.5%) were in the low risk group. Of the 104 patients with a tumor size of 2–5 cm, 69 (66.3%) were in the low risk group and 35 (33.7%) were in the intermediate risk group. The 53 patients with a tumor size of >5 cm varied in risk, with 24 (45.3%) in the low risk group, 10 (18.9%) in the intermediate risk group, and 19 (35.8%) in the high risk group.
Tumor recurrence
During an average follow-up period of 67 months (0.2–200 months), recurrence was observed in 7 patients (3.5%). There was 1 case of recurrence in the very low risk group in a patient with a tumor size of 1–2 cm, and 2 cases in the very low risk group in patients with tumor sizes of 2–5 cm. In the high risk group, there were 3 cases of recurrences in patients with tumors >5 cm (5.7%). Table 2 shows the clinical characteristics of the 7 patients who developed a recurrence. The most common sites of recurrence were the stomach and liver. All the patients were subsequently treated with imatinib. There was 1 death during the follow-up period, of a patient in the high risk group with a tumor size of ≥10cm, and the cause of death was GI bleeding due to GIST. The 5-year recurrence-free survival rates were 100% in the very low risk group, 98.9% in the low risk group, 95.5% in the intermediate risk group, and 86.7% in the high risk group; these differences were statistically significant (log-rank test, p <0.05) (Fig. 2).
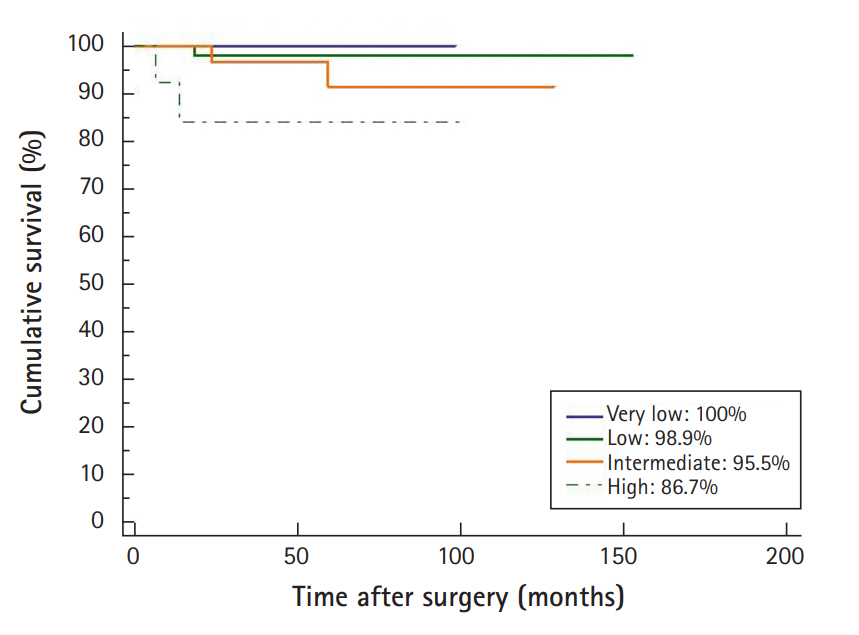
The 5-year recurrence-free survival rates according to risk groups
The recurrence-free survival rates in the very low, low, intermediate, and high risk groups were 100%, 98.9%, 95.5%, and 86.7%, respectively. Statistical analysis showed a significant lower recurrence-free survival rate in the high risk group (log-rank test, p <0.05
Discussion
This study was a retrospective analysis of recurrence in patients who underwent surgery for gastric GIST. Compared to overseas studies, in which the mean age of gastric GIST patients was around 63 years and there were more male patients than female patients [10,11], the mean age in our study was 60.8 years, and there was a predominance of female patients. Other Korean studies have also reported younger patient ages (around 60 years on average) and more females than overseas studies [12,13].
The first aim of treatment for gastric GIST is complete surgical resection with no infiltration of the margin. The desired resection margin has been reported to be 1–2 cm [14]. However, recent studies have reported that extensive resection to ensure a wide margin is not necessary, and is unrelated to good surgical outcomes [15,16]. Therefore, the objectives of gastric GIST surgery have shifted recently toward only complete resection with a clear margin, and since lymph node metastasis is extremely rare, lymph node dissection is not generally performed [7].
The postoperative recurrence rates of gastric GIST reported in Western studies is 17–24% [17,18]. However, studies from Korea and Japan report lower postoperative recurrence rates. Similarly, we observed a postoperative recurrence rate of 3.5%, albeit in a cohort with a large number of patients in the very low and low risk groups. Other Korean studies have also reported similar recurrence rates. Yang et al. [19] reported recurrence in 6 out of 105 patients (5.7%) after surgical resection, and Kim et al. [7,20] reported postoperative recurrence rates of 2.7% and 4.8%. Japanese studies have shown even lower recurrence rates; for example, Honda et al. [21] reported recurrence in only 1 out of 78 patients (1.3%) after laparoscopic surgery for gastric GIST. These low recurrence rates are thought to be due to recent improvements in surgical techniques and early diagnosis. In particular, Korea and Japan have high rates of early gastric cancer endoscopic examinations for early detection are performed more often than in Western countries. As a result, smaller GISTs are more likely to be found.
The 5-year recurrence-free survival rates in the very low and low risk groups in our study were 100% and 98.9%, respectively, which were similar to other studies [5]. In addition, there were no deaths during the follow-up period in the low risk group or below, which is also consistent with other studies [22]. However, the high risk group showed a low 5-year recurrence-free survival rate of 86.7%, and there was also a case of death due to GIST-related bleeding in the high risk group. This is also the same as other studies, with high risk GIST patients showing high recurrence rates and poor prognoses; the 5-year survival rate after complete resection of high risk GIST has been reported in the range of 35–70% [23-25].
Our study had several limitations. First, as a retrospective study, the numbers of patients in the very low, low, intermediate, and high risk groups are all different, and the follow-up duration and timing of follow-up examinations also differ. Second, there were not many patients who underwent endoscopic ultrasound to determine the risk of gastric GIST. In particular, 1 case of recurrence was observed even among patients with a tumor size of <2cm, which is generally managed by monitoring without surgery. Had the internal appearance of the tumor been inspected by endoscopic ultrasound, it might have been possible to analyze the risk of recurrence in more detail.
In conclusion, although the recurrence rate after complete resection of gastric GIST is very low, the high risk group requires thorough postoperative follow-up to monitor for recurrence. In addition, even in the very low risk group, with tumor size <2cm and low mitotic index, cases of recurrence do occur, and so regular postoperative follow-up is essential.
Notes
Conflict of interest
All authors declare no conflicts-of-interest related to this article.

